- Molybdenum
-
niobium ← molybdenum → technetium Cr
↑
Mo
↓
WAppearance gray metallic

General properties Name, symbol, number molybdenum, Mo, 42 Pronunciation /ˌmɒlɪbˈdiːnəm/ mol-ib-dee-nəm
or /məˈlɪbdɨnəm/ mə-lib-di-nəmElement category transition metal Group, period, block 6, 5, d Standard atomic weight 95.94(1) Electron configuration [Kr] 5s1 4d5 Electrons per shell 2, 8, 18, 13, 1 (Image) Physical properties Phase solid Density (near r.t.) 10.28 g·cm−3 Liquid density at m.p. 9.33 g·cm−3 Melting point 2896 K, 2623 °C, 4753 °F Boiling point 4912 K, 4639 °C, 8382 °F Heat of fusion 37.48 kJ·mol−1 Heat of vaporization 598 kJ·mol−1 Molar heat capacity 24.06 J·mol−1·K−1 Vapor pressure P (Pa) 1 10 100 1 k 10 k 100 k at T (K) 2742 2994 3312 3707 4212 4879 Atomic properties Oxidation states 6, 5, 4, 3, 2, 1[1], -1, -2
(strongly acidic oxide)Electronegativity 2.16 (Pauling scale) Ionization energies 1st: 684.3 kJ·mol−1 2nd: 1560 kJ·mol−1 3rd: 2618 kJ·mol−1 Atomic radius 139 pm Covalent radius 154±5 pm Miscellanea Crystal structure body-centered cubic Magnetic ordering paramagnetic[2] Electrical resistivity (20 °C) 53.4 nΩ·m Thermal conductivity 138 W·m−1·K−1 Thermal expansion (25 °C) 4.8 µm·m−1·K−1 Young's modulus 329 GPa Shear modulus 126 GPa Bulk modulus 230 GPa Poisson ratio 0.31 Mohs hardness 5.5 Vickers hardness 1530 MPa Brinell hardness 1500 MPa CAS registry number 7439-98-7 Most stable isotopes Main article: Isotopes of molybdenum iso NA half-life DM DE (MeV) DP 92Mo 14.84% 92Mo is stable with 50 neutrons 93Mo syn 4×103 y ε - 93Nb 94Mo 9.25% 94Mo is stable with 52 neutrons 95Mo 15.92% 95Mo is stable with 53 neutrons 96Mo 16.68% 96Mo is stable with 54 neutrons 97Mo 9.55% 97Mo is stable with 55 neutrons 98Mo 24.13% 98Mo is stable with 56 neutrons 99Mo syn 65.94 h β− 0.436, 1.214 99mTc γ 0.74, 0.36,
0.14- 100Mo 9.63% 7.8×1018 y β−β− 3.04 100Ru mol-ib-dee-nəm or /məˈlɪbdɨnəm/ mə-lib-di-nəm), is a Group 6 chemical element with the symbol Mo and atomic number 42. The name is from Neo-Latin Molybdaenum, from Ancient Greek Μόλυβδος molybdos, meaning lead, itself proposed as a loanword from Anatolian Luvian and Lydian languages,[3] since its ores were confused with lead ores.[4] The free element, which is a silvery metal, has the sixth-highest melting point of any element. It readily forms hard, stable carbides, and for this reason it is often used in high-strength steel alloys. Molybdenum does not occur as a free metal on Earth, but rather in various oxidation states in minerals. Industrially, molybdenum compounds are used in high-pressure and high-temperature applications, as pigments and catalysts. Molybdenum minerals have long been known, but the element was "discovered" (in the sense of differentiating it as a new entity from the mineral salts of other metals) in 1778 by Carl Wilhelm Scheele. The metal was first isolated in 1781 by Peter Jacob Hjelm.
Most molybdenum compounds have low solubility in water, but the molybdate ion MoO42− is soluble and forms when molybdenum-containing minerals are in contact with oxygen and water.
Molybdenum-containing enzymes are used as catalysts by some bacteria to break the chemical bond in atmospheric molecular nitrogen, allowing biological nitrogen fixation. At least 50 molybdenum-containing enzymes are now known in bacteria and animals, though only the bacterial and cyanobacterial enzymes are involved in nitrogen fixation. Owing to the diverse functions of the remainder of the enzymes, molybdenum is a required element for life in higher organisms (eukaryotes), though not in all bacteria.
Contents
Characteristics
Physical properties
In its pure form, molybdenum is a silvery-white metal with a Mohs hardness of 5.5. It has a melting point of 2,623 °C (4,753 °F); of the naturally occurring elements, only tantalum, osmium, rhenium, tungsten, and carbon have higher melting points.[4] Molybdenum burns only at temperatures above 600 °C (1,112 °F).[5] It has one of the lowest coefficients of thermal expansion among commercially used metals.[6] The tensile strength of molybdenum wires increases about 3 times, from about 10 to 30 GPa, when their diameter decreases from ~50–100 nm to 10 nm.[7]
Isotopes
Main article: Isotopes of molybdenumThere are 35 known isotopes of molybdenum, ranging in atomic mass from 83 to 117, as well as four metastable nuclear isomers. Seven isotopes occur naturally, with atomic masses of 92, 94, 95, 96, 97, 98, and 100. Of these naturally occurring isotopes, only molybdenum-100 is unstable.[8] All unstable isotopes of molybdenum decay into isotopes of niobium, technetium, and ruthenium.[9]
Molybdenum-98 is the most abundant isotope, comprising 24.14% of all molybdenum. Molybdenum-100 has a half-life of about 1019 y and undergoes double beta decay into ruthenium-100. Molybdenum isotopes with mass numbers from 111 to 117 all have half-lives of approximately 150 ns.[8][9]
As also noted below, the most common isotopic molybdenum application involves molybdenum-99, which is a fission product. It is a parent radioisotope to the short-lived gamma-emitting daughter radioisotope technetium-99m, a nuclear isomer used in various imaging applications in medicine.[10] In 2008, the Delft University of Technology applied for a patent on the molybdenum-98-based production of technetium-99 [1]
Compounds and chemistry
See also Category: Molybdenum compounds.Oxidation
stateExample[11] −2 Na2[Mo2(CO)10] 0 Mo(CO)6 +1 Na[C6H6Mo] +2 MoCl2 +3 Na3[Mo(CN)]6 +4 MoS2 +5 MoCl5 +6 Molybdenum is a transition metal with an electronegativity of 1.8 on the Pauling scale and an atomic mass of 95.94 g/mol.[12] It does not visibly react with oxygen or water at room temperature, and the bulk oxidation occurs at temperatures above 790 °C, resulting in molybdenum trioxide:
- 2 Mo + 3 O2 → 2 MoO3
The trioxide is volatile and sublimates at high temperatures. This prevents formation of a continuous protective oxide layer, which would stop the bulk oxidation of metal.[13] Molybdenum has several oxidation states, the most stable being +4 and +6 (bolded in the table). The chemistry and the compounds show more similarity to those of tungsten than that of chromium. An example is the instability of molybdenum(III) and tungsten(III) compounds as compared with the stability of the chromium(III) compounds. The highest oxidation state is common in the molybdenum(VI) oxide (MoO3), whereas the normal sulfur compound is molybdenum disulfide MoS2.[14]
Molybdenum(VI) oxide is soluble in strong alkaline water, forming molybdates (MoO42−). Molybdates are weaker oxidants than chromates, but they show a similar tendency to form complex oxyanions by condensation at lower pH values, such as [Mo7O24]6− and [Mo8O26]4−. Polymolybdates can incorporate other ions into their structure, forming polyoxometalates.[15] The dark-blue phosphorus-containing heteropolymolybdate P[Mo12O40]3− is used for the spectroscopic detection of phosphorus.[16] The broad range of oxidation states of molybdenum is reflected in various molybdenum chlorides:[14]
- Molybdenum(II) chloride MoCl2 (yellow solid)
- Molybdenum(III) chloride MoCl3 (dark red solid)
- Molybdenum(IV) chloride MoCl4 (black solid)
- Molybdenum(V) chloride MoCl5 (dark green solid)
- Molybdenum(VI) chloride MoCl6 (brown solid)
The structure of the MoCl2 is composed of Mo6Cl84+ clusters with four chloride ions to compensate the charge.[14]
Like chromium and some other transition metals, molybdenum is able to form quadruple bonds, such as in Mo2(CH3COO)4. This compound can be transformed into Mo2Cl84−, which also has a quadruple bond.[14]
The oxidation state 0 is possible with carbon monoxide as ligand, such as in molybdenum hexacarbonyl, Mo(CO)6.[14]
History
Molybdenite—the principal ore from which molybdenum is now extracted—was previously known as molybdena. Molybdena was confused with and often implemented as though it were graphite. Like graphite, molybdenite can be used to blacken a surface or as a solid lubricant.[17] Even when molybdena was distinguishable from graphite, it was still confused with a common lead ore (now called galena), which took its name from Ancient Greek Μόλυβδος molybdos, meaning lead.[6] Although apparent deliberate alloying of molybdenum with steel in one 14th-century Japanese sword (mfd. ca. 1330) has been reported, that art was never employed widely and was later lost.[18] In 1754, Bengt Andersson Qvist examined molybdenite and determined that it did not contain lead and was thus not the same as galena.[19]
It was not until 1778 that Swedish chemist Carl Wilhelm Scheele realized that molybdena was neither graphite nor lead.[20][21] He and other chemists then correctly assumed that it was the ore of a distinct new element, named molybdenum for the mineral in which it was discovered. Peter Jacob Hjelm successfully isolated molybdenum by using carbon and linseed oil in 1781.[6][22]
For about a century after its isolation, molybdenum had no industrial use, owing to its relative scarcity, difficulty extracting the pure metal, and the immaturity of the metallurgical subfield.[23][24][25] Early molybdenum steel alloys showed great promise in their increased hardness, but efforts were hampered by inconsistent results and a tendency toward brittleness and recrystallization. In 1906, William D. Coolidge filed a patent for rendering molybdenum ductile, leading to its use as a heating element for high-temperature furnaces and as a support for tungsten-filament light bulbs; oxide formation and degradation require that moly be physically sealed or held in an inert gas. In 1913, Frank E. Elmore developed a flotation process to recover molybdenite from ores; flotation remains the primary isolation process.
During the first World War, demand for molybdenum spiked; it was used both in armor plating and as a substitute for tungsten in high speed steels. Some British tanks were protected by 75 mm (3 in) manganese steel plating, but this proved to be ineffective. The manganese steel plates were replaced with 25 mm (1 in) molybdenum-steel plating allowing for higher speed, greater maneuverability, and better protection.[6] After the war, demand plummeted until metallurgical advances allowed extensive development of peacetime applications. In World War II, molybdenum again saw strategic importance as a substitute for tungsten in steel alloys.[26]
Occurrence
Molybdenite on quartz
The world's largest producers of molybdenum materials are the United States, China, Chile, Peru and Canada.[6][27][28] [29][29] Though molybdenum is found in such minerals as wulfenite (PbMoO4) and powellite (CaMoO4), the main commercial source of molybdenum is molybdenite (MoS2). Molybdenum is mined as a principal ore, and is also recovered as a byproduct of copper and tungsten mining.[4] Large mines in Colorado (such as the Henderson mine and the now-inactive Climax mine)[30] and in British Columbia yield molybdenite as their primary product, while many porphyry copper deposits such as the Bingham Canyon Mine in Utah and the Chuquicamata mine in northern Chile produce molybdenum as a byproduct of copper mining.
The Knaben mine in southern Norway was opened in 1885, making it the first molybdenum mine. It remained open until 1973.[31]
Molybdenum is the 54th most abundant element in the Earth's crust and the 25th most abundant element in the oceans, with an average of 10 parts per billion; it is the 42nd most abundant element in the Universe.[5][6] The Russian Luna 24 mission discovered a molybdenum-bearing grain (1 × 0.6 µm) in a pyroxene fragment taken from Mare Crisium on the Moon.[32]
Production
In molybdenite processing, the molybdenite is first heated to a temperature of 700 °C (1,292 °F) and the sulfide is oxidized into molybdenum(VI) oxide by air:[14]
- 2 MoS2 + 7 O2 → 2 MoO3 + 4 SO2
The oxidized ore is then either heated to 1,100 °C (2,010 °F) to sublimate the oxide, or leached with ammonia which reacts with the molybdenum(VI) oxide to form water-soluble molybdates:
- MoO3 + 2 NH4OH → (NH4)2(MoO4) + H2O
Copper, an impurity in molybdenite, is less soluble in ammonia. To completely remove it from the solution, it is precipitated with hydrogen sulfide.[14]
Pure molybdenum is produced by reduction of the oxide with hydrogen, while the molybdenum for steel production is reduced by the aluminothermic reaction with addition of iron to produce ferromolybdenum. A common form of ferromolybdenum contains 60% molybdenum.[6][14]
Molybdenum has a value of approximately $30,000 per tonne as of August 2009. It maintained a price at or near $10,000 per tonne from 1997 through 2003, and reached, due to increased demand, a peak of $103,000 per tonne in June 2005.[33] In 2008 the London Metal Exchange announced that molybdenum would be traded as a commodity on the exchange.[34]
Applications
 MoSi2 heating element
MoSi2 heating element
Alloys
The ability of molybdenum to withstand extreme temperatures without significantly expanding or softening makes it useful in applications that involve intense heat, including the manufacture of armour, aircraft parts, electrical contacts, industrial motors and filaments.[6][35]
Most high-strength steel alloys (example 41xx steels) contain 0.25% to 8% molybdenum.[4] Despite such small portions, more than 43,000 tonnes of molybdenum are used as an alloying agent each year in stainless steels, tool steels, cast irons and high-temperature superalloys.[5]
Molybdenum is also used in steel alloys for its high corrosion resistance and weldability.[5][27] Molybdenum contributes further corrosion resistance to "chrome-moly" type-300 stainless steels (high-chromium steels that are corrosion-resistant already due to their chromium content) and especially so in the so-called superaustenitic stainless steels (such as alloy AL-6XN). Molybdenum acts by increasing lattice strain, thus increasing the energy required to dissolve out iron atoms from the surface.
Because of its lower density and more stable price, molybdenum is sometimes used instead of tungsten.[5] An example is the 'M' series of high-speed steels such as M2, M4 and M42 as substitution for the 'T' steel series which contain tungsten. Molybdenum can be implemented both as an alloying agent and as a flame-resistant coating for other metals. Although its melting point is 2,623 °C (4,753 °F), molybdenum rapidly oxidizes at temperatures above 760 °C (1,400 °F) making it better-suited for use in vacuum environments.[35]
TZM (Mo (~99%), Ti (~0.5%), Zr (~0.08%) and some C) is a corrosion-resisting molybdenum superalloy that resists molten fluoride salts at temperatures above 1300C. It has about twice the strength of pure Mo, and is more ductile and more weldable, yet in tests it resisted corrosion of a standard eutectic salt (FLiBe) and salt vapors used in molten salt reactors for 1100 hours with so little corrosion that it was difficult to measure.[36][37]
Other molybdenum-based alloys which do not contain iron have only limited applications. For example, because of the corrosion resistance against molten zinc, both pure molybdenum and the molybdenum/tungsten alloy (70%/30%) are used for piping, stirrers and pump impellers which come into contact with molten zinc.[38]
Other applications as catalyst and compounds
- Molybdenum-99 is a parent radioisotope to the daughter radioisotope technetium-99m, which is used in many medical procedures.[39]
- Molybdenum disulfide (MoS2) is used as a solid lubricant and a high-pressure high-temperature (HPHT) antiwear agent. It forms strong films on metallic surfaces and is a common additive to HPHT greases—in case of a catastrophic grease failure, thin layer of molybdenum prevents contact of the lubricated parts.[40] It also has semiconducting properties with distinct advantages over traditional silicon or graphene in electronics applications.[41] MoS2 is also used as a catalyst in hydrocracking of petroleum fractions which contain nitrogen, sulfur and oxygen.[42]
- Molybdenum disilicide (MoSi2) is an electrically conducting ceramic with primary use in heating elements operating at temperatures above 1500 °C in air.[43]
- Molybdenum trioxide (MoO3) is used as an adhesive between enamels and metals.[20] Lead molybdate (wulfenite) co-precipitated with lead chromate and lead sulfate is a bright-orange pigment used with ceramics and plastics.[44]
- Molybdenum powder is used as a fertilizer for some plants, such as cauliflower.[5]
- The element is also used in NO, NO2, NOx analyzers in power plants for pollution controls. At 350 °C (662 °F) the element acts as a catalyst for NO2/NOx to form only NO molecules for consistent readings by infrared light.[45]
- Ammonium heptamolybdate is used in biological staining procedures.
Biological role
Biochemistry
The most important role of the molybdenum in living organisms is as a metal heteroatom at the active site in certain enzymes. In nitrogen fixation in certain bacteria, the nitrogenase enzyme, which is involved in the terminal step of reducing molecular nitrogen, usually contains molybdenum in the active site (though replacement of Mo with iron or vanadium is also known). The structure of the catalytic center of the enzyme is similar to that in iron-sulfur proteins: it incorporates a Fe4S3 and multiple MoFe3S3 clusters.[46]
In 2008, evidence was reported that a scarcity of molybdenum in the Earth's early oceans was a limiting factor for nearly two billion years in the further evolution of eukaryotic life (which includes all plants and animals) as eukaryotes cannot fix nitrogen, and must therefore acquire most of their organic nitrogen from prokaryotic bacteria.[47][48][49] The scarcity of molybdenum resulted from the relative lack of oxygen in the early ocean. However, once oxygen dissolved in seawater it helped dissolve molybdenum from minerals on the sea bottom, making it available to nitrogen-fixing bacteria and allowing them to provide more nitrogen for higher forms of life.
Although oxygen once promoted nitrogen fixation via making molybdenum available in water, it also directly poisons nitrogenase enzymes, so that historically, after oxygen arrived in large quantities in Earth's air and water, organisms which continued to fix nitrogen in aerobic conditions were required to isolate their nitrogen-fixing enzymes in heterocysts, or similar structures which protect them from too much oxygen.
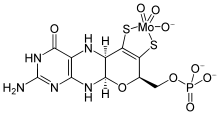 The molybdenum cofactor (pictured) contains an organic complex called molybdopterin, which binds an oxidized molybdenum through adjacent sulfur (or occasionally selenium) atoms.
The molybdenum cofactor (pictured) contains an organic complex called molybdopterin, which binds an oxidized molybdenum through adjacent sulfur (or occasionally selenium) atoms.
Though molybdenum forms compounds with various organic molecules, including carbohydrates and amino acids, it is transported throughout the human body as MoO42−.[50] At least 50 molybdenum-containing enzymes were known by 2002, mostly in bacteria, and their number is increasing with every year;[51][52] those enzymes include aldehyde oxidase, sulfite oxidase and xanthine oxidase.[6] In some animals, and in humans, the oxidation of xanthine to uric acid, a process of purine catabolism, is catalyzed by xanthine oxidase, a molybdenum-containing enzyme. The activity of xanthine oxidase is directly proportional to the amount of molybdenum in the body. However, an extremely high concentration of molybdenum reverses the trend and can act as an inhibitor in both purine catabolism and other processes. Molybdenum concentrations also affect protein synthesis, metabolism and growth.[50]
In animals and plants these enzymes use molybdenum bound at the active site in a tricyclic molybdenum cofactor. All molybdenum-using enzymes so far identified in nature use this cofactor, save for the phylogenetically ancient molybdenum nitrogenases, which fix nitrogen in some bacteria and cyanobacteria.[53] Molybdenum enzymes in plants and animals catalyze the oxidation and sometimes reduction of certain small molecules, as part of the regulation of nitrogen, sulfur and carbon cycles.[54]
Human dietary intake and deficiency
The human body contains about 0.07 mg of molybdenum per kilogram of weight.[55] It occurs in higher concentrations in the liver and kidneys and in lower concentrations in the vertebrae.[5] Molybdenum is also present within human tooth enamel and may help prevent its decay.[56]
The average daily intake of molybdenum varies between 0.12 and 0.24 mg, but it depends on the molybdenum content of the food.[57] Pork, lamb and beef liver each have approximately 1.5 parts per million of molybdenum. Other significant dietary sources include green beans, eggs, sunflower seeds, wheat flour, lentils, cucumbers and cereal grain.[6] Acute toxicity has not been seen in humans, and the toxicity depends strongly on the chemical state. Studies on rats show a median lethal dose (LD50) as low as 180 mg/kg for some Mo compounds.[58] Although human toxicity data is unavailable, animal studies have shown that chronic ingestion of more than 10 mg/day of molybdenum can cause diarrhea, growth retardation, infertility, low birth weight and gout; it can also affect the lungs, kidneys and liver.[57][59] Sodium tungstate is a competitive inhibitor of molybdenum. Dietary tungsten reduces the concentration of molybdenum in tissues.[5]
Dietary molybdenum deficiency from low soil concentration of molybdenum has been associated with increased rates of esophageal cancer in a geographical band from northern China to Iran.[60][61] Compared to the United States, which has a greater supply of molybdenum in the soil, people living in these areas have about 16 times greater risk for esophageal squamous cell carcinoma.[62]
Molybdenum deficiency has also been reported as a consequence of non-molybdenum supplemented total parenteral nutrition (complete intravenous feeding) for long periods of time. It results in high blood levels of sulfite and urate, in much the same way as molybdenum cofactor deficiency. However, presumably since pure molybdenum deficiency from this mechanism is seen primarily in adults, the neurological consequences have not been as marked as for the congenital cofactor deficiency.
Related diseases
A congenital molybdenum cofactor deficiency disease, seen in infants, results in interference with the ability of the body to use molybdenum in enzymes. It causes high levels of sulphite and urate, and neurological damage.[63][64] The cause is the inability of the body to synthesize molybdenum cofactor, a heterocyclic molecule which binds molybdenum at the active site in all known human enzymes which use molybdenum.
Copper-molybdenum antagonism
High levels of molybdenum can interfere with the body's uptake of copper, producing copper deficiency. Molybdenum prevents plasma proteins from binding to copper, and it also increases the amount of copper that is excreted in urine. Ruminants that consume high amounts of molybdenum develop symptoms including diarrhea, stunted growth, anemia and achromotrichia (loss of hair pigment). These symptoms can be alleviated by the administration of more copper into the system, both in dietary form and by injection.[65] The condition, as an effective copper deficiency, can be aggravated by excess sulfur.[5][66]
Copper reduction or deficiency can also be deliberately induced for therapeutic purposes by the compound ammonium tetrathiomolybdate, in which the bright red anion tetrathiomolybdate is the copper-chelating agent. Tetrathiomolybdate was first used therapeutically in the treatment of copper toxicosis in animals. It was then introduced as a treatment in Wilson's disease, a hereditary copper metabolism disorder in humans; it acts both by competing with copper absorption in the bowel and by increasing excretion. It has also been found to have an inhibitory effect on angiogenesis, potentially via the inhibition of copper ion dependent membrane translocation process invovling a non-classical secretion pathway.[67] This makes it an interesting investigatory treatment for cancer, age-related macular degeneration, and other diseases featuring excessive blood vessel deposition.[68][69]
Precautions
Molybdenum dusts and fumes, which can be generated by mining or metalworking, can be toxic, especially if ingested (including dust trapped in the sinuses and later swallowed).[58] Low levels of prolonged exposure can cause irritation to the eyes and skin. Direct inhalation or ingestion of molybdenum and its oxides should be avoided.[70][71] OSHA regulations specify the maximum permissible molybdenum exposure in an 8-hour day as 5 mg/m3. Chronic exposure to 60 to 600 mg/m3 can cause symptoms including fatigue, headaches and joint pains.[72]
References
- ^ "Molybdenum: molybdenum(I) fluoride compound data". OpenMOPAC.net. http://openmopac.net/data_normal/molybdenum(i)%20fluoride_jmol.html. Retrieved 2007-12-10.
- ^ Magnetic susceptibility of the elements and inorganic compounds, in Handbook of Chemistry and Physics 81st edition, CRC press.
- ^ Melchert, Craig. "Greek mólybdos as a Loanword from Lydian". University of North Carolina at Chapel Hill. http://www.unc.edu/~melchert/molybdos.pdf. Retrieved 2011-04-23.
- ^ a b c d editor-in-chief David R. Lide. (1994). "Molybdenum". In Lide, David R.. CRC Handbook of Chemistry and Physics. 4. Chemical Rubber Publishing Company. p. 18. ISBN 0849304741.
- ^ a b c d e f g h i edited by Glenn D. Considine. (2005). "Molybdenum". In Considine, Glenn D.. Van Nostrand's Encyclopedia of Chemistry. New York: Wiley-Interscience. pp. 1038–1040. ISBN 9780471615255.
- ^ a b c d e f g h i j Emsley, John (2001). Nature's Building Blocks. Oxford: Oxford University Press. pp. 262–266. ISBN 0198503415. http://books.google.com/?id=j-Xu07p3cKwC&pg=PA265.
- ^ Shpak, Anatoly P; Kotrechko, Sergiy O; Mazilova, Tatjana I; Mikhailovskij, Igor M (2009). "Inherent tensile strength of molybdenum nanocrystals". Science and Technology of Advanced Materials 10 (4): 045004. Bibcode 2009STAdM..10d5004S. doi:10.1088/1468-6996/10/4/045004.
- ^ a b Audi, Georges (2003). "The NUBASE Evaluation of Nuclear and Decay Properties". Nuclear Physics A (Atomic Mass Data Center) 729: 3–128. Bibcode 2003NuPhA.729....3A. doi:10.1016/j.nuclphysa.2003.11.001.
- ^ a b editor-in-chief, David R. Lide. (2006). Lide, David R.. ed. CRC Handbook of Chemistry and Physics. 11. CRC. pp. 87–88. ISBN 0849304873.
- ^ Armstrong, John T. (2003). "Technetium". Chemical & Engineering News. http://pubs.acs.org/cen/80th/technetium.html. Retrieved 2009-07-07.
- ^ Schmidt, Max (1968). "VI. Nebengruppe" (in German). Anorganische Chemie II.. Wissenschaftsverlag. pp. 119–127.
- ^ "Properties of Molybdenum". Integral Scientist Periodic Table. Qivx, Inc.. 2003. http://www.qivx.com/ispt/elements/ptw_042.php. Retrieved 2007-06-10.
- ^ Davis, Joseph R. (1997). Heat-resistant materials. ASM International. p. 365. ISBN 0871705966. http://books.google.com/books?id=GEHA8_bix0oC&pg=PA365.
- ^ a b c d e f g h Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils (1985). Lehrbuch der Anorganischen Chemie (91–100 ed.). Walter de Gruyter. pp. 1096–1104. ISBN 3-11-007511-3.
- ^ Pope, Michael T.; Müller, Achim (1997). "Polyoxometalate Chemistry: An Old Field with New Dimensions in Several Disciplines". Angewandte Chemie International Edition 30: 34. doi:10.1002/anie.199100341.
- ^ ed. by Nollet, Leo M.L. (2000). Handbook of water analysis. New York, NY: Marcel Dekker. pp. 280–288. ISBN 9780824784331. http://books.google.com/?id=YZpW4Y4Q_PIC&pg=PA280.
- ^ Lansdown, A.R. (1999). Molybdenum disulphide lubrication. 35. Elsevier. ISBN 9780444500328.
- ^ International Molybdenum Association, "Molybdenum History"
- ^ Van der Krogt, Peter (2006-01-10). "Molybdenum". Elementymology & Elements Multidict. http://elements.vanderkrogt.net/element.php?sym=Mo. Retrieved 2007-05-20.
- ^ a b Gagnon, Steve. "Molybdenum". Jefferson Science Associates, LLC. http://education.jlab.org/itselemental/ele042.html. Retrieved 2007-05-06.
- ^ Scheele, C. W. K. (1779). "Versuche mit Wasserbley;Molybdaena". Svenska vetensk. Academ. Handlingar 40: 238. http://resolver.sub.uni-goettingen.de/purl?PPN324352840_0040.
- ^ Hjelm, P. J. (1788). "Versuche mit Molybdäna, und Reduction der selben Erde". Svenska vetensk. Academ. Handlingar 49: 268. http://resolver.sub.uni-goettingen.de/purl?PPN324352840_0009_02_NS.
- ^ Hoyt, Samuel Leslie (1921). Metallography, Volume 2. McGraw-Hill.
- ^ Krupp, Alfred; Wildberger, Andreas (1888). The metallic alloys: A practical guide for the manufacture of all kinds of alloys, amalgams, and solders, used by metal-workers ... with an appendix on the coloring of alloys. H.C. Baird & Co.. pp. 60.
- ^ Gupta, C.K. (1992). Extractive Metallurgy of Molybdenum. CRC Press. ISBN 9780849347580.
- ^ Millholland, Ray (1941-08). "Battle of the Billions: American industry mobilizes machines, materials, and men for a job as big as digging 40 Panama Canals in one year". Popular Science: pp. 61.
- ^ a b "Molybdenum Statistics and Information". U.S. Geological Survey. 2007-05-10. http://minerals.usgs.gov/minerals/pubs/commodity/molybdenum/. Retrieved 2007-05-10.
- ^ Lide, David R., ed (2006). CRC Handbook of Chemistry and Physics. 4. Chemical Rubber Publishing Company. pp. 22–23. ISBN 0849304873.
- ^ a b Magyar, Michael J.. "Commodity Summary 2008:Molybdenum". United States Geological Survey. http://minerals.usgs.gov/minerals/pubs/commodity/molybdenum/mcs-2008-molyb.pdf. Retrieved 2010-04-01.
- ^ Coffman, Paul B. (1937). "The Rise of a New Metal: The Growth and Success of the Climax Molybdenum Company". The Journal of Business of the University of Chicago 10: 30. doi:10.1086/232443.
- ^ Langedal, M (1997). "Dispersion of tailings in the Knabena—Kvina drainage basin, Norway, 1: Evaluation of overbank sediments as sampling medium for regional geochemical mapping". Journal of Geochemical Exploration 58 (2–3): 157. doi:10.1016/S0375-6742(96)00069-6.
- ^ Jambor, J.L. et al. (2002). "New mineral names". American Mineralogist 87: 181. http://www.minsocam.org/msa/AmMin/TOC/Abstracts/2002_Abstracts/Jan02_Abstracts/Jambor_p181_02.pdf.
- ^ "Dynamic Prices and Charts for Molybdenum". InfoMine Inc.. 2007. http://www.infomine.com/investment/metalschart.asp?c=molybdenum&u=mt&submit1=Display+Chart&x=usd&r=15y. Retrieved 2007-05-07.
- ^ "LME to launch minor metals contracts in H2 2009". London Metal Exchange. 4 September 2008. http://lme.com/6241.asp. Retrieved 28 July 2009.
- ^ a b "Molybdenum". AZoM.com Pty. Limited. 2007. http://www.azom.com/details.asp?ArticleID=616. Retrieved 2007-05-06.
- ^ Smallwood, Robert E. (1984). "TZM Moly Alloy". ASTM special technical publication 849: Refractory metals and their industrial applications: a symposium. ASTM International. p. 9. ISBN 19849780803102033. http://books.google.com/?id=agaacIr25KcC&pg=PA9.
- ^ "Compatibility of Molybdenum-Base Alloy TZM, with LiF-BeF2-ThF4-UF4". Oak Ridge National Laboratory Report. 1969-12. http://www.energyfromthorium.com/forum/download/file.php?id=805. Retrieved 2010-09-02.
- ^ Cubberly, W. H.; Bakerjian, Ramon (1989). Tool and manufacturing engineers handbook. Society of Manufacturing Engineers. p. 421. ISBN 9780872633513. http://books.google.com/?id=NRXnXmFRjWYC&pg=PT421.
- ^ Gottschalk, A (1969). "Technetium-99m in clinical nuclear medicine". Annual review of medicine 20: 131–40. doi:10.1146/annurev.me.20.020169.001023. PMID 4894500.
- ^ Winer, W. (1967). "Molybdenum disulfide as a lubricant: A review of the fundamental knowledge". Wear 10 (6): 422. doi:10.1016/0043-1648(67)90187-1.
- ^ "New transistors: An alternative to silicon and better than graphene". Physorg.com. January 30, 2011. http://www.physorg.com/news/2011-01-transistors-alternative-silicon-graphene.html. Retrieved January 30, 2011.
- ^ Topsøe, H.; Clausen, B. S.; Massoth, F. E. (1996). Hydrotreating Catalysis, Science and Technology. Berlin: Springer-Verlag.
- ^ Moulson, A. J.; Herbert, J. M. (2003). Electroceramics: materials, properties, applications. John Wiley and Sons. p. 141. ISBN 0471497487. http://books.google.com/?id=FbMfaqSgOxsC&pg=PA141.
- ^ International Molybdenum Association
- ^ Lal, S.; Patil, R. S. (2001). "Monitoring of atmospheric behaviour of NOx from vehicular traffic". Environmental Monitoring and Assessment 68 (1): 37–50. doi:10.1023/A:1010730821844. PMID 11336410.
- ^ Dos Santos, Patricia C.; Dean, Dennis R.; Dean, Dennis R. (2008). "A newly discovered role for iron-sulfur clusters". PNAS 105 (33): 11589–11590. Bibcode 2008PNAS..10511589D. doi:10.1073/pnas.0805713105. PMC 2575256. PMID 18697949. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2575256.
- ^ Scott, C; Lyons, T. W.; Bekker, A.; Shen, Y.; Poulton, S. W.; Chu, X.; Anbar, A. D. (2008). "Tracing the stepwise oxygenation of the Proterozoic ocean". Nature 452 (7186): 456–460. Bibcode 2008Natur.452..456S. doi:10.1038/nature06811. PMID 18368114.
- ^ "International team of scientists discover clue to delay of life on Earth". Eurekalert.org. http://www.eurekalert.org/pub_releases/2008-03/asu-ito032508.php. Retrieved 2008-10-25.
- ^ "Scientists uncover the source of an almost 2 billion year delay in animal evolution". Eurekalert.org. http://www.eurekalert.org/pub_releases/2008-03/nu-sut032508.php. Retrieved 2008-10-25.
- ^ a b Mitchell, Phillip C. H. (2003). "Overview of Environment Database". International Molybdenum Association. http://www.hse.imoa.info/Default.asp?page=110. Retrieved 2007-05-05.
- ^ Enemark, John H. et al. (2004). "Synthetic Analogues and Reaction Systems Relevant to the Molybdenum and Tungsten Oxotransferases". Chem. Rev. 104 (2): 1175–1200. doi:10.1021/cr020609d. PMID 14871153.
- ^ Mendel, RR; Bittner, F (2006). "Cell biology of molybdenum". Biochimica et Biophysica Acta 1763 (7): 621–635. doi:10.1016/j.bbamcr.2006.03.013. PMID 16784786.
- ^ Fischer, B; Enemark, JH; Basu, P (1998). "A chemical approach to systematically designate the pyranopterin centers of molybdenum and tungsten enzymes and synthetic models". Journal of inorganic biochemistry 72 (1–2): 13–21. PMID 9861725.. Summarized in MetaCyc Compound: molybdopterin. Accessed Nov. 16, 2009.
- ^ Kisker, C.; Schindelin, H.; Baas, D.; Rétey, J.; Meckenstock, R.U; Kroneck, P.M.H (1999). "A structural comparison of molybdenum cofactor-containing enzymes". FEMS Microbiol. Rev. 22 (5): 503–521. doi:10.1111/j.1574-6976.1998.tb00384.x. PMID 9990727.
- ^ Holleman, Arnold F.; Wiberg, Egon (2001). Inorganic chemistry. Academic Press. p. 1384. ISBN 0123526515. http://books.google.com/?id=vEwj1WZKThEC&pg=PA1384.
- ^ Curzon, M. E. J.; Kubota, J.; Bibby, B.G. (1971). "Environmental Effects of Molybdenum on Caries" (PDF). Journal of Dental Research 50 (1): 74–77. doi:10.1177/00220345710500013401. http://jdr.sagepub.com/cgi/reprint/50/1/74.pdf.
- ^ a b Coughlan, M. P. (1983). "The role of molybdenum in human biology". Journal of Inherited Metabolic Disease 6: 70–77. doi:10.1007/BF01811327. PMID 6312191.
- ^ a b "Risk Assessment Information System: Toxicity Summary for Molybdenum". Oak Ridge National Laboratory. Archived from the original on September 19, 2007. http://web.archive.org/web/20070919204536/http://rais.ornl.gov/tox/profiles/molybdenum_f_V1.shtml. Retrieved 2008-04-23.
- ^ Barceloux, Donald G.; Barceloux, Donald (1999). "Molybdenum". Clinical Toxicology 37 (2): 231–237. doi:10.1081/CLT-100102422. PMID 10382558.
- ^ Yang, Chung S. (1980). "Research on Esophageal Cancer in China: a Review". Cancer Research 40 (8 Pt 1): 2633. PMID 6992989. http://cancerres.aacrjournals.org/cgi/reprint/40/8_Part_1/2633.pdf.
- ^ Nouri, Mohsen; Chalian, Hamid; Bahman, Atiyeh; Mollahajian, Hamid; Ahmadi-Faghih, Mohammadamin; Fakheri, Hafez; Soroush, Ahmadreza (2008). "Nail Molybdenum and Zinc Contents in Populations with Low and Moderate Incidence of Esophageal Cancer". Archives of Iranian Medicine 11: 392. http://www.ams.ac.ir/AIM/08114/0010.pdf.
- ^ Taylor, Philip R.; Li, Bing; Dawsey, Sanford M.; Li, Jun-Yao; Yang, Chung S.; Guo, Wande; Blot, William J. (1994). "Prevention of Esophageal Cancer: The Nutrition Intervention Trials in Linxian, China". Cancer Research 54 (7 Suppl): 2029s–2031s. PMID 8137333. http://www.nutritionhealthinfo.com/nutrition/nutrition_0163_001.pdf.
- ^ Smolinsky, B. et al. (2008). "Splice-specific Functions of Gephyrin in Molybdenum Cofactor Biosynthesis". Journal of Biological Chemistry 283 (25): 17370–9. doi:10.1074/jbc.M800985200. PMID 18411266. http://www.jbc.org/content/283/25/17370.full.
- ^ Reiss, J. (2000). "Genetics of molybdenum cofactor deficiency". Human Genetics 106 (2): 157–63. doi:10.1007/s004390051023. PMID 10746556.
- ^ Suttle, N. F. (1974). "Recent studies of the copper-molybdenum antagonism". Proceedings of the Nutrition Society (CABI Publishing) 33 (3): 299–305. doi:10.1079/PNS19740053. PMID 4617883.
- ^ Hauer,Gerald Copper deficiency in cattle. Bison Producers of Alberta. Accessed Dec. 16, 2010.
- ^ Nickel, W (2003). "The Mystery of nonclassical protein secretion, a current view on cargo proteins and potential export routes". Eur. J. Biochem. 270 (10): 2109–2119. doi:10.1046/j.1432-1033.2003.03577.x. PMID 12752430.
- ^ Brewer GJ et al. (2003). "Treatment of Wilson disease with ammonium tetrathiomolybdate: III. Initial therapy in a total of 55 neurologically affected patients and follow-up with zinc therapy". Arch Neurol 60 (3): 379–85. doi:10.1001/archneur.60.3.379. PMID 12633149. http://archneur.ama-assn.org/cgi/content/full/60/3/379.
- ^ Brewer, GJ; Dick, RD; Grover, DK; Leclaire, V; Tseng, M; Wicha, M; Pienta, K; Redman, BG et al. (2000). "Treatment of metastatic cancer with tetrathiomolybdate, an anticopper, antiangiogenic agent: Phase I study". Clinical cancer research : an official journal of the American Association for Cancer Research 6 (1): 1–10. PMID 10656425.
- ^ "Material Safety Data Sheet – Molybdenum". The REMBAR Company, Inc.. 2000-09-19. Archived from the original on March 23, 2007. http://web.archive.org/web/20070323103727/http://www.rembar.com/MSDSmo.htm. Retrieved 2007-05-13.
- ^ "Material Safety Data Sheet – Molybdenum Powder". CERAC, Inc.. 1994-02-23. http://asp.cerac.com/CatalogNet/default.aspx?p=msdsFile&msds=m000121.htm. Retrieved 2007-10-19.
- ^ "NIOSH Documentation for ILDHs Molybdenum". National Institute for Occupational Safety and Health. 1996-08-16. http://www.cdc.gov/niosh/idlh/moly-mo.html. Retrieved 2007-05-31.
External links
- Mineral & Exploration – Map of World Molybdenum Producers 2009
- "Mining A Mountain" Popular Mechanics, July 1935 pp.63-64
Periodic table H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Rf Db Sg Bh Hs Mt Ds Rg Cn Uut Uuq Uup Uuh Uus Uuo Alkali metals Alkaline earth metals Lanthanides Actinides Transition metals Other metals Metalloids Other nonmetals Halogens Noble gases Unknown chem. properties Large version Molybdenum compounds Categories:- Chemical elements
- Dietary minerals
- Molybdenum
- Transition metals
- Refractory metals
- Biology and pharmacology of chemical elements
Wikimedia Foundation. 2010.
Look at other dictionaries:
molybdenum — Symbol: Mo Atomic number: 42 Atomic weight: 95.94 Silvery white, hard metallic transition element. It is chemically unreactive and is not affected by most acids. It oxidizes at high temperatures. There are seven natural isotopes, and four… … Elements of periodic system
Molybdenum — Mol yb*de num, n. [NL.: cf. F. molybd[ e]ne. See {Molybdena}.] (Chem.) A rare element of the chromium group, occurring in nature in the minerals molybdenite and wulfenite, and when reduced obtained as a hard, silver white, difficulty fusible… … The Collaborative International Dictionary of English
molybdenum — (n.) metallic element, 1816, from molybdena, used generally for lead like minerals, from Gk. molybdos lead, also black graphite, related to L. plumbum lead (see PLUMB (Cf. plumb) (n.)), and like it probably borrowed from a lost Mediterranean… … Etymology dictionary
molybdenum — [mə lib′də nəm] n. [ModL: so named (1781) by K. W. Scheele (see SCHEELITE) after its isolation by P. J. Hjelm (1746 1813), Swed chemist < molybdaena, molybdenite, term used because of resemblance to lead ore < L molybdaena, lead, galena… … English World dictionary
molybdenum — /meuh lib deuh neuhm/, n. Chem. a silver white metallic element, used as an alloy with iron in making hard, high speed cutting tools. Symbol: Mo; at. wt.: 95.94; at. no.: 42; sp. gr.: 10.2. [1810 20; < NL, alter. of earlier molybdena < L… … Universalium
molybdenum — noun Etymology: New Latin, from molybdena, a lead ore, molybdenite, molybdenum, from Latin molybdaena galena, from Greek molybdaina, from molybdos lead Date: 1814 a metallic element that resembles chromium and tungsten in many properties, is used … New Collegiate Dictionary
molybdenum — A silvery white metallic element, atomic no. 42, atomic wt. 95.94; a bioelement found in a number of proteins ( e.g., xanthine oxidase). See m. target tube. [G. molybdaina, a piece of lead; a … Medical dictionary
molybdenum-99 — A reactor produced radioisotope of molybdenum with a half life of 2.7476 days, used in radionuclide generators for the production of technetium 99m … Medical dictionary
molybdenum — мед. молибден (molybdenum) Микроэлемент. Способствует метаболизму жиров и углеводов. Жизненно важен для усвоения железа. Участвует в производстве мочевой кислоты. см. тж микроэлементы … Универсальный дополнительный практический толковый словарь И. Мостицкого
molybdenum — molibdenas statusas T sritis chemija apibrėžtis Cheminis elementas. simbolis( iai) Mo atitikmenys: lot. molybdaenum angl. molybdenum rus. молибден … Chemijos terminų aiškinamasis žodynas


