- Chromium
-
This article is about the chemical element. For other uses, see Chromium (disambiguation).
vanadium ← chromium → manganese -
↑
Cr
↓
MoAppearance silvery metallic

General properties Name, symbol, number chromium, Cr, 24 Pronunciation /ˈkroʊmiəm/ kroh-mee-əm Element category transition metal Group, period, block 6, 4, d Standard atomic weight 51.9961(6) Electron configuration [Ar] 3d5 4s1 Electrons per shell 2, 8, 13, 1 (Image) Physical properties Phase solid Density (near r.t.) 7.19 g·cm−3 Liquid density at m.p. 6.3 g·cm−3 Melting point 2180 K, 1907 °C, 3465 °F Boiling point 2944 K, 2671 °C, 4840 °F Heat of fusion 21.0 kJ·mol−1 Heat of vaporization 339.5 kJ·mol−1 Molar heat capacity 23.35 J·mol−1·K−1 Vapor pressure P (Pa) 1 10 100 1 k 10 k 100 k at T (K) 1656 1807 1991 2223 2530 2942 Atomic properties Oxidation states 6, 5, 4, 3, 2, 1, -1, -2
(strongly acidic oxide)Electronegativity 1.66 (Pauling scale) Ionization energies
(more)1st: 652.9 kJ·mol−1 2nd: 1590.6 kJ·mol−1 3rd: 2987 kJ·mol−1 Atomic radius 128 pm Covalent radius 139±5 pm Miscellanea Crystal structure body-centered cubic Magnetic ordering AFM (rather: SDW[1]) Electrical resistivity (20 °C) 125 nΩ·m Thermal conductivity 93.9 W·m−1·K−1 Thermal expansion (25 °C) 4.9 µm·m−1·K−1 Speed of sound (thin rod) (20 °C) 5940 m·s−1 Young's modulus 279 GPa Shear modulus 115 GPa Bulk modulus 160 GPa Poisson ratio 0.21 Mohs hardness 8.5 Vickers hardness 1060 MPa Brinell hardness 1120 MPa CAS registry number 7440-47-3 Most stable isotopes Main article: Isotopes of chromium iso NA half-life DM DE (MeV) DP 50Cr 4.345% > 1.8×1017y εε - 50Ti 51Cr syn 27.7025 d ε - 51V γ 0.320 - 52Cr 83.789% 52Cr is stable with 28 neutrons 53Cr 9.501% 53Cr is stable with 29 neutrons 54Cr 2.365% 54Cr is stable with 30 neutrons Chromium (
 /ˈkroʊmiəm/ kroh-mee-əm) is a chemical element which has the symbol Cr and atomic number 24. It is the first element in Group 6. It is a steely-gray, lustrous, hard metal that takes a high polish and has a high melting point. It is also odorless, tasteless, and malleable. The name of the element is derived from the Greek word "chrōma" (χρώμα), meaning colour,[2] because many of its compounds are intensely coloured. Chromium oxide was used by the Chinese in the Qin dynasty over 2,000 years ago to coat weapons such as bronze crossbow bolts and steel swords found at the Terracotta Army. It later came to the attention of the west when it was discovered by Louis Nicolas Vauquelin in the mineral crocoite (lead(II) chromate) in 1797. Crocoite was used as a pigment, and after the discovery that the mineral chromite also contains chromium, this latter mineral was used to produce pigments as well.
/ˈkroʊmiəm/ kroh-mee-əm) is a chemical element which has the symbol Cr and atomic number 24. It is the first element in Group 6. It is a steely-gray, lustrous, hard metal that takes a high polish and has a high melting point. It is also odorless, tasteless, and malleable. The name of the element is derived from the Greek word "chrōma" (χρώμα), meaning colour,[2] because many of its compounds are intensely coloured. Chromium oxide was used by the Chinese in the Qin dynasty over 2,000 years ago to coat weapons such as bronze crossbow bolts and steel swords found at the Terracotta Army. It later came to the attention of the west when it was discovered by Louis Nicolas Vauquelin in the mineral crocoite (lead(II) chromate) in 1797. Crocoite was used as a pigment, and after the discovery that the mineral chromite also contains chromium, this latter mineral was used to produce pigments as well.Chromium was regarded with great interest because of its high corrosion resistance and hardness. A major development was the discovery that steel could be made highly resistant to corrosion and discoloration by adding chromium to form stainless steel. This application, along with chrome plating (electroplating with chromium) are currently the highest-volume uses of the metal. Chromium and ferrochromium are produced from the single commercially viable ore, chromite, by silicothermic or aluminothermic reaction or by roasting and leaching processes.
Although trivalent chromium (Cr(III)) is required in trace amounts for sugar and lipid metabolism, few cases have been reported where its complete removal from the diet has caused chromium deficiency. In larger amounts and different forms chromium can be toxic and carcinogenic. The most prominent example of toxic chromium is hexavalent chromium (Cr(VI)). Abandoned chromium production sites often require environmental cleanup.
Contents
Characteristics
Physical
Chromium is remarkable for its magnetic properties: it is the only elemental solid which shows antiferromagnetic ordering at room temperature (and below). Above 38 °C, it transforms into a paramagnetic state.[1]
Passivation
Chromium metal left standing in air is passivated by oxygen, forming a thin protective oxide surface layer. This layer is a spinel structure only a few atoms thick. It is very dense, and prevents the diffusion of oxygen into the underlying material. This barrier is in contrast to iron or plain carbon steels, where the oxygen migrates into the underlying material and causes rusting.[3] The passivation can be enhanced by short contact with oxidizing acids like nitric acid. Passivated chromium is stable against acids. The opposite effect can be achieved by treatment with a strong reducing reactant that destroys the protective oxide layer on the metal. Chromium metal treated in this way readily dissolves in weak acids.[4]
Chromium, unlike metals such as iron and nickel, does not suffer from hydrogen embrittlement. However, it does suffer from nitrogen embrittlement, reacting with nitrogen from air and forming brittle nitrides at the high temperatures necessary to work the metal parts.[5]
Occurrence
Chromium is the 21st most abundant element in Earth's crust with an average concentration of 100 ppm.[6] Chromium compounds are found in the environment, due to erosion of chromium-containing rocks and can be distributed by volcanic eruptions. The concentrations range in soil is between 1 and 3000 mg/kg, in sea water 5 to 800 µg/liter, and in rivers and lakes 26 µg/liter to 5.2 mg/liter.[7]
 Crocoite (PbCrO4)
Crocoite (PbCrO4)
 Chromite ore
Chromite ore
Chromium is mined as chromite (FeCr2O4) ore.[8] About two-fifths of the chromite ores and concentrates in the world are produced in South Africa, while Kazakhstan, India, Russia, and Turkey are also substantial producers. Untapped chromite deposits are plentiful, but geographically concentrated in Kazakhstan and southern Africa.[9]
Although rare, deposits of native chromium exist.[10][11] The Udachnaya Pipe in Russia produces samples of the native metal. This mine is a kimberlite pipe, rich in diamonds, and the reducing environment helped produce both elemental chromium and diamond.[12]
The relation between Cr(III) and Cr(VI) strongly depends on pH and oxidative properties of the location, but in most cases, the Cr(III) is the dominating species,[7] although in some areas the ground water can contain up to 39 µg/liter of total chromium of which 30 µg/liter is present as Cr(VI).[13]
Isotopes
Main article: Isotopes of chromiumNaturally occurring chromium is composed of three stable isotopes; 52Cr, 53Cr and 54Cr with 52Cr being the most abundant (83.789% natural abundance). 19 radioisotopes have been characterized with the most stable being 50Cr with a half-life of (more than) 1.8×1017 years, and 51Cr with a half-life of 27.7 days. All of the remaining radioactive isotopes have half-lives that are less than 24 hours and the majority of these have half-lives that are less than 1 minute. This element also has 2 meta states.[14]
53Cr is the radiogenic decay product of 53Mn. Chromium isotopic contents are typically combined with manganese isotopic contents and have found application in isotope geology. Mn-Cr isotope ratios reinforce the evidence from 26Al and 107Pd for the early history of the solar system. Variations in 53Cr/52Cr and Mn/Cr ratios from several meteorites indicate an initial 53Mn/55Mn ratio that suggests Mn-Cr isotopic composition must result from in-situ decay of 53Mn in differentiated planetary bodies. Hence 53Cr provides additional evidence for nucleosynthetic processes immediately before coalescence of the solar system.[15]
The isotopes of chromium range in atomic mass from 43 u (43Cr) to 67 u (67Cr). The primary decay mode before the most abundant stable isotope, 52Cr, is electron capture and the primary mode after is beta decay.[14] 53Cr has been posited as a proxy for atmospheric oxygen concentration.[16]
Compounds
Oxidation
states[note 1][17][18]−2 Na2[Cr(CO)5] −1 Na2[Cr2(CO)10] 0 Cr(C6H6)2 +1 K3[Cr(CN)5NO] +2 CrCl2 +3 CrCl3 +4 K2CrF6 +5 K3CrO8 +6 K2CrO4 Chromium is a member of the transition metals, in group 6. Chromium(0) has an electronic configuration of 4s13d5, owing to the lower energy of the high spin configuration. Chromium exhibits a wide range of possible oxidation states, where the +3 state is most stable energetically; the +3 and +6 states are most commonly observed in chromium compounds, whereas the +1, +4 and +5 states are rare.[17][18]
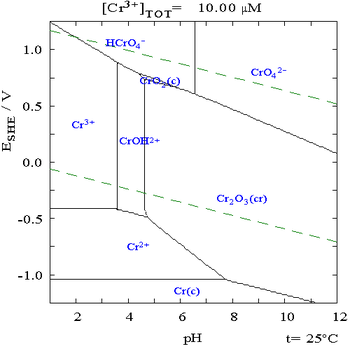
The following is the Pourbaix diagram for chromium in pure water, perchloric acid or sodium hydroxide:[7][19]
Chromium(III)
A large number of chromium(III) compounds are known. Chromium(III) can be obtained by dissolving elemental chromium in acids like hydrochloric acid or sulfuric acid. The Cr3+ ion has a similar radius (63 pm) to the Al3+ ion (radius 50 pm), so they can replace each other in some compounds, such as in chrome alum and alum. When a trace amount of Cr3+ replaces Al3+ in corundum (aluminium oxide, Al2O3), the red-colored ruby is formed.
Chromium(III) ions tend to adopt octahedral molecular geometry, with six ligands. The colors of these solutions is determined by the ligands attached to the Cr centre. The commercially available chromium(III) chloride hydrate is the dark green complex [CrCl2(H2O)4]Cl, but two other forms are known: pale green [CrCl(H2O)5]Cl2, and the violet [Cr(H2O)6]Cl3. If water-free green chromium(III) chloride is dissolved in water then the green solution turns violet after some time, due to the substitution of water for chloride in the inner coordination sphere. This kind of reaction is also observed in chrome alum solutions and other water-soluble chromium(III) salts. The reverse reaction may be induced by heating the solution.
Chromium(III) hydroxide (Cr(OH)3) is amphoteric, dissolving in acidic solutions to form [Cr(H2O)6]3+, and in basic solutions to form [Cr(OH)6]3−. It is dehydrated by heating to form the green chromium(III) oxide (Cr2O3), which is the stable oxide with a crystal structure identical to that of corundum.[4]
Chromium(VI)
Chromium(VI) compounds are powerful oxidants at low or neutral pH. Most important are chromate anion (CrO2−
4) and dichromate (Cr2O72-) anions, which exist in equilibrium:- 2 [CrO4]2- + 2 H+
 [Cr2O7]2- + 2 H2O
[Cr2O7]2- + 2 H2O
Chromium(VI) halides are known also and include hexafluoride CrF6 and chromyl chloride (CrO2Cl2).[4]
Sodium chromate is produced industrially by the oxidative roasting of chromite ore with calcium or sodium carbonate. The dominant species is therefore, by the law of mass action, determined by the pH of the solution. The change in equilibrium is visible by a change from yellow (chromate) to orange (dichromate), such as when an acid is added to a neutral solution of potassium chromate. At yet lower pH values, further condensation to more complex oxyanions of chromium is possible.
Both the chromate and dichromate anions are strong oxidizing reagents at low pH:[4]
Sodium chromate (Na2CrO4)
- Cr2O2−
7 + 14 H3O+ + 6 e− → 2 Cr3+ + 21 H2O (ε0 = 1.33 V)
They are, however, only moderately oxidizing at high pH:[4]
- CrO2−
4 + 4 H2O + 3 e− → Cr(OH)3 + 5 OH− (ε0 = −0.13 V)
Chromium(VI) compounds in solution can be detected by adding an acidic hydrogen peroxide solution. The unstable dark blue chromium(VI) peroxide (CrO5) is formed, which can be stabilized as an ether adduct CrO5·OR2.[4]
Chromic acid has the hypothetical formula H2CrO4. It is a vaguely described chemical, despite many well-defined chromates and dichromates are known. The dark red chromium(VI) oxide CrO3, the acid anhydride of chromic acid, is sold industrially as "chromic acid".[4] It can be produced by mixing sulfuric acid with dichromate, and is a strong oxidizing agent.
In 2010, the Environmental Working Group studied the drinking water in 35 American cities. The study was the first nationwide analysis measuring the presence of the chemical in U.S. water systems. The study found measurable hexavalent chromium in the tap water of 31 of the cities sampled, with Norman, Oklahoma, at the top of list; 25 cities had levels that exceeded California's proposed limit.[20] Note: Concentrations of Cr VI in US municipal drinking water supplies reported by EWG are within likely, natural background levels for the areas tested and not necessarily indicative of industrial pollution (CalEPA Fact Sheet), as asserted by EWG. This factor was not taken into consideration in their report.
Chromium(IV) and chromium(V)
The oxidation state +5 is only realized in few compounds but are intermediates in many reactions involving oxidations by chromate. The only binary compound is the volatile chromium(V) fluoride (CrF5). This red solid has a melting point of 30 °C and a boiling point of 117 °C. It can be synthesized by treating chromium metal with fluorine at 400 °C and 200 bar pressure. The peroxochromate(V) is another example of the +5 oxidation state. Potassium peroxochromate (K3[Cr(O2)4]) is made by reacting potassium chromate with hydrogen peroxide at low temperatures. This red brown compound is stable at room temperature but decomposes spontaneously at 150–170 °C.[21]
Compounds of chromium(IV) (in the +4 oxidation state) are slightly more common than those of chromium(V). The tetrahalides, CrF4, CrCl4, and CrBr4, can be produced by treating the trihalides (CrX3) with the corresponding halogen at elevated temperatures. Such compounds are susceptible to disproportionation reactions and are not stable in water.
Chromium(I) and chromium(II)
Many chromium(II) compounds are known, including the water-stable chromium(II) chloride, CrCl2, which can be made by reduction of chromium(III) chloride with zinc. The resulting bright blue solution is only stable at neutral pH.[4] Many chromous carboxylates are also known, most famously, the red chromous acetate (Cr2(O2CCH3)4), which features a quadruple bond. As verified by X-ray diffraction, a Cr-Cr quintuple bond (length 183.51(4) pm) has also been described.[22] Extremely bulky monodentate ligands stabilize this compound by shielding the quintuple bond from further reactions.
Chromium(0)
Main article: organochromium chemistryMany chromium(0) compounds are known. Most are derivatives of chromium hexacarbonyl or bis(benzene)chromium.
History
Weapons found in burial pits dating from the late 3rd century BC Qin Dynasty of the Terracotta Army near Xi'an, China have been analyzed by archaeologists. Although buried more than 2,000 years ago, the ancient bronze tips of crossbow bolts and swords found at the site showed no sign of corrosion, because the bronze was coated with chromium.[23]
Chromium later came to the attention of westerners in the 18th century. On 26 July 1761, Johann Gottlob Lehmann found an orange-red mineral in the Beryozovskoye mines in the Ural Mountains which he named Siberian red lead. Though misidentified as a lead compound with selenium and iron components, the mineral was Crocoite (lead chromate) with a formula of PbCrO4.[24]
In 1770, Peter Simon Pallas visited the same site as Lehmann and found a red lead mineral that had useful properties as a pigment in paints. The use of Siberian red lead as a paint pigment developed rapidly. A bright yellow pigment made from crocoite also became fashionable.[24]
In 1797, Louis Nicolas Vauquelin received samples of crocoite ore. He produced chromium trioxide (CrO3) by mixing crocoite with hydrochloric acid. In 1798, Vauquelin discovered that he could isolate metallic chromium by heating the oxide in a charcoal oven.[25] He was also able to detect traces of chromium in precious gemstones, such as ruby or emerald.[24][26]
During the 1800s, chromium was primarily used as a component of paints and in tanning salts. At first, crocoite from Russia was the main source, but in 1827, a larger chromite deposit was discovered near Baltimore, United States. This made the United states the largest producer of chromium products till 1848 when large deposits of chromite where found near Bursa, Turkey.[8]
Chromium is also known for its luster when polished. It is used as a protective and decorative coating on car parts, plumbing fixtures, furniture parts and many other items, usually applied by electroplating. Chromium was used for electroplating as early as 1848, but this use only became widespread with the development of an improved process in 1924.[27]
Metal alloys now account for 85% of the use of chromium. The remainder is used in the chemical industry and refractory and foundry industries.
Production
 Piece of chromium produced with aluminothermic reaction
Piece of chromium produced with aluminothermic reaction
Approximately 4.4 million metric tons of marketable chromite ore were produced in 2000, and converted into ~3.3 million tons of ferro-chrome with an approximate market value of 2.5 billion United States dollars.[28] The largest producers of chromium ore have been South Africa (44%) India (18%), Kazakhstan (16%) Zimbabwe (5%), Finland (4%) Iran (4%) and Brazil (2%) with several other countries producing the rest of less than 10% of the world production.[28]
The two main products of chromium ore refining are ferrochromium and metallic chromium. For those products the ore smelter process differs considerably. For the production of ferrochromium, the chromite ore (FeCr2O4) is reduced in large scale in electric arc furnace or in smaller smelters with either aluminium or silicon in an aluminothermic reaction.[29]
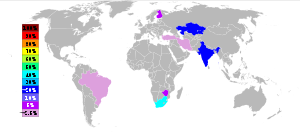 Chromium ore output in 2002[28]
Chromium ore output in 2002[28]
For the production of pure chromium, the iron has to be separated from the chromium in a two step roasting and leaching process. The chromite ore is heated with a mixture of calcium carbonate and sodium carbonate in the presence of air. The chromium is oxidized to the hexavalent form, while the iron forms the stable Fe2O3. The subsequent leaching at higher elevated temperatures dissolves the chromates and leaves the insoluble iron oxide. The chromate is converted by sulfuric acid into the dichromate.[29]
- 4 FeCr2O4 + 8 Na2CO3 + 7 O2 → 8 Na2CrO4 + 2 Fe2O3 + 8 CO2
- 2 Na2CrO4 + H2SO4 → Na2Cr2O7 + Na2SO4 + H2O
The dichromate is converted to the chromium(III) oxide by reduction with carbon and then reduced in an aluminothermic reaction to chromium.[29]
- Na2Cr2O7 + 2 C → Cr2O3 + Na2CO3 + CO
- Cr2O3 + 2 Al → Al2O3 + 2 Cr
Applications
Metallurgy
The strengthening effect of forming stable metal carbides at the grain boundaries and the strong increase in corrosion resistance made chromium an important alloying material for steel. The high speed tool steels contain between 3 and 5% chromium. Stainless steel, the main corrosion-proof metal alloy, is formed when chromium is added to iron in sufficient concentrations, usually above 11%. For its formation, ferrochromium is added to the molten iron. Also nickel-based alloys increase in strength due to the formation of discrete, stable metal carbide particles at the grain boundaries. For example, Inconel 718 contains 18.6% chromium. Because of the excellent high temperature properties of these nickel superalloys, they are used in jet engines and gas turbines in lieu of common structural materials.[30]
The relative high hardness and corrosion resistance of unalloyed chromium makes it a good surface coating, being still the most "popular" metal coating with unbeatable combined durability. A thin layer of chromium is deposited on pretreated metallic surfaces by electroplating techniques. There are two deposition methods: Thin, below 1 µm thickness, layers are deposited by chrome plating, and are used for decorative surfaces. If wear-resistant surfaces are needed then thicker chromium layers are deposited. Both methods normally use acidic chromate or dichromate solutions. To prevent the energy consuming change in oxidation state, the use of Chromium(III) sulfate is under development, but for most applications, the established process is used.[27]
In the chromate conversion coating process, the strong oxidative properties of chromates are used to deposit a protective oxide layer on metals like aluminium, zinc and cadmium. This passivation and the self healing properties by the chromate stored in the chromate conversion coating, which is able to migrate to local defects, are the benefits of this coating method.[31] Because of environmental and health regulations on chromates, alternative coating method are under development.[32]
Anodizing of aluminium is another electrochemical process, which does not lead to the deposition of chromium, but uses chromic acid as electrolyte in the solution. During anodization, an oxide layer is formed on the aluminium. The use of chromic acid, instead of the normally used sulfuric acid, leads to a slight difference of these oxide layers.[33] The high toxicity of Cr(VI) compounds, used in the established chromium electroplating process, and the strengthening of safety and environmental regulations demand a search for substitutes for chromium or at least a change to less toxic chromium(III) compounds.[27]
Dye and pigment
The mineral crocoite (lead chromate PbCrO4) was used as a yellow pigment shortly after its discovery. After a synthesis method became available starting from the more abundant chromite, chrome yellow was, together with cadmium yellow, one of the most used yellow pigments. The pigment does not photo degrade and has a strong color, and was used for school buses in the US and for Postal Service (for example Deutsche Post) in Europe. The use of chrome yellow declined due to environmental and safety concerns and was replaced by organic pigments or other lead-free alternatives. Other pigments based on chromium are, for example, the bright red pigment chrome red, which is a basic lead chromate (PbCrO4·Pb(OH)2). Chrome green is a mixture of Prussian blue and chrome yellow, while the chrome oxide green is Chromium(III) oxide.[35]
A red color is achieved by doping chromium(III) into the crystals of corundum, which are then called ruby. Therefore, chromium is used in producing synthetic rubies.[36]
Chromium oxides are also used as a green color in glassmaking and as a glaze in ceramics.[37]
Wood preservative
Because of their toxicity, chromium(VI) salts are used for the preservation of wood. For example, chromated copper arsenate (CCA) is used in timber treatment to protect wood from decay fungi, wood attacking insects, including termites, and marine borers.[38] The formulations contain chromium based on the oxide CrO3 between 35.3% and 65.5%. In the United States, 65,300 metric tons of CCA solution have been used in 1996.[38]
Tanning
Main article: TanningChromium(III) salts, especially chrome alum and chromium(III) sulfate, are used in the tanning of leather. The chromium(III) stabilizes the leather by cross linking the collagen fibers.[39] Chromium tanned leather can contain between 4 and 5% of chromium, which is tightly bound to the proteins.[8] Although the form of chromium used for tanning is not the toxic hexavalent variety, there remains interest in management of chromium in the tanning industry such as recovery and reuse, direct/indirect recycling,[40] use of less chromium or "chrome-less" tanning are practised to better manage chromium in tanning.
Refractory material
The high heat resistivity and high melting point makes chromite and chromium(III) oxide a material for high temperature refractory applications, like blast furnaces, cement kilns, molds for the firing of bricks and as foundry sands for the casting of metals. In these applications, the refractory materials are made from mixtures of chromite and magnesite. The use is declining because of the environmental regulations due to the possibility of the formation of chromium(VI).[29]
Catalysts
Several chromium compounds are used as catalysts for processing hydrocarbons. For example the Phillips catalysts for the production of polyethylene are mixtures of chromium and silicon dioxide or mixtures of chromium and titanium and aluminium oxide.[41] Fe-Cr mixed oxides are employed as high-temperature catalysts for the water gas shift reaction.[42][43] Copper chromite is a useful hydrogenation catalyst.[44]
Other use
- Chromium(IV) oxide (CrO2) is a magnetic compound. Its ideal shape anisotropy, which imparts high coercivity and remanent magnetization, made it a compound superior to the γ-Fe2O3. Chromium(IV) oxide is used to manufacture magnetic tape used in high-performance audio tape and standard audio cassettes.[45] Chromates can prevent corrosion of steel under wet conditions, and therefore chromates are added to drilling muds.[46]
- Chromium(III) oxide is a metal polish known as green rouge.
- Chromic acid is a powerful oxidizing agent and is a useful compound for cleaning laboratory glassware of any trace of organic compounds. It is prepared in situ by dissolving potassium dichromate in concentrated sulfuric acid, which is then used to wash the apparatus. Sodium dichromate is sometimes used because of its higher solubility (50 g/L versus 200 g/L respectively). Potassium dichromate is a chemical reagent, used in cleaning laboratory glassware and as a titrating agent. It is also used as a mordant (i.e., a fixing agent) for dyes in fabric.
Biological role
Chromium has no verified biological role and has been classified as not essential for mammals.[47] (Cr(III) or Cr3+) occurs in trace amounts and appears to be benign.[48] Chromium deficiency is controversial or is at least extremely rare. It has been attributed to only three people on parenteral nutrition, which is when a patient is fed a liquid diet through intravenous drips.[49] In contrast, hexavalent chromium (Cr(VI) or Cr6+) is very toxic and mutagenic when inhaled. Cr(VI) has not been established as a carcinogen when in solution, although it may cause allergic contact dermatitis (ACD).[50] Although no biological role for chromium has ever been demonstrated, dietary supplements for chromium include chromium(III) picolinate, chromium(III) polynicotinate, and related materials. The benefit of those supplements is still under investigation and is questioned by some studies.[51][52]
The use of chromium-containing dietary supplements is controversial owing to the absence of any verified biological role, the expense of these supplements, and the complex effects of their use.[53] The popular dietary supplement chromium picolinate complex generates chromosome damage in hamster cells.[54] In the United States the dietary guidelines for daily chromium uptake were lowered from 50–200 µg for an adult to 35 µg (adult male) and to 25 µg (adult female).[55]
Precautions
Main article: Chromium toxicityWater insoluble chromium(III) compounds and chromium metal are not considered a health hazard, while the toxicity and carcinogenic properties of chromium(VI) have been known for a long time.[56]
Because of the specific transport mechanisms, only limited amounts of chromium(III) enter the cells. Several in vitro studies indicated that high concentrations of chromium(III) in the cell can lead to DNA damage.[57] Acute oral toxicity ranges between 1.5 and 3.3 mg/kg.[58] The proposed beneficial effects of chromium(III) and the use as dietary supplements yielded some controversial results, but recent reviews suggest that moderate uptake of chromium(III) through dietary supplements poses no risk.[57]
The acute oral toxicity for chromium(VI) ranges between 50 and 150 µg/kg.[58] In the body, chromium(VI) is reduced by several mechanisms to chromium(III) already in the blood before it enters the cells. The chromium(III) is excreted from the body, whereas the chromate ion is transferred into the cell by a transport mechanism, by which also sulfate and phosphate ions enter the cell. The acute toxicity of chromium(VI) is due to its strong oxidational properties. After it reaches the blood stream, it damages the kidneys, the liver and blood cells through oxidation reactions. Hemolysis, renal and liver failure are the results of these damages. Aggressive dialysis can improve the situation.[59]
The carcinogenity of chromate dust is known for a long time, and in 1890 the first publication described the elevated cancer risk of workers in a chromate dye company.[60][61] Three mechanisms have been proposed to describe the genotoxicity of chromium(VI). The first mechanism includes highly reactive hydroxyl radicals and other reactive radicals which are by products of the reduction of chromium(VI) to chromium(III). The second process includes the direct binding of chromium(V), produced by reduction in the cell, and chromium(IV) compounds to the DNA. The last mechanism attributed the genotoxicity to the binding to the DNA of the end product of the chromium(III) reduction.[62]
Chromium salts (chromates) are also the cause of allergic reactions in some people. Chromates are often used to manufacture, amongst other things, leather products, paints, cement, mortar and anti-corrosives. Contact with products containing chromates can lead to allergic contact dermatitis and irritant dermatitis, resulting in ulceration of the skin, sometimes referred to as "chrome ulcers". This condition is often found in workers that have been exposed to strong chromate solutions in electroplating, tanning and chrome-producing manufacturers.[63][64]
Environmental issues
As chromium compounds were used in dyes and paints and the tanning of leather, these compounds are often found in soil and groundwater at abandoned industrial sites, now needing environmental cleanup and remediation per the treatment of brownfield land. Primer paint containing hexavalent chromium is still widely used for aerospace and automobile refinishing applications.[65]
See also
- Chromium compounds
- Chromium minerals
Notes
- ^ Most common oxidation states of chromium are in bold. The right column lists one representative compound for each oxidation state.
References
- ^ a b Fawcett, Eric (1988). "Spin-density-wave antiferromagnetism in chromium". Reviews of Modern Physics 60: 209. Bibcode 1988RvMP...60..209F. doi:10.1103/RevModPhys.60.209.
- ^ χρώμα, Henry George Liddell, Robert Scott, A Greek-English Lexicon, on Perseus
- ^ Wallwork, G. R. (1976). "The oxidation of alloys". Reports on the Progress Physics 39 (5): 401–485. Bibcode 1976RPPh...39..401W. doi:10.1088/0034-4885/39/5/001. http://www.iop.org/EJ/article/0034-4885/39/5/001/rpv39i5p401.pdf.
- ^ a b c d e f g h Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils; (1985). "Chromium" (in German). Lehrbuch der Anorganischen Chemie (91–100 ed.). Walter de Gruyter. pp. 1081–1095. ISBN 3110075113.
- ^ National Research Council (U.S.). Committee on Coatings (1970). High-temperature oxidation-resistant coatings: coatings for protection from oxidation of superalloys, refractory metals, and graphite. National Academy of Sciences. ISBN 0309017696. http://books.google.com/?id=CGMrAAAAYAAJ.
- ^ Emsley, John (2001). "Chromium". Nature's Building Blocks: An A-Z Guide to the Elements. Oxford, England, UK: Oxford University Press. pp. 495–498. ISBN 0198503407.
- ^ a b c Kotaś, J.; Stasicka, Z (2000). "Chromium occurrence in the environment and methods of its speciation". Environmental Pollution 107 (3): 263–283. doi:10.1016/S0269-7491(99)00168-2. PMID 15092973.
- ^ a b c National Research Council (U.S.). Committee on Biologic Effects of Atmospheric Pollutants (1974). Chromium. National Academy of Sciences. pp. 155. ISBN 9780309022170. http://books.google.com/?id=ZZsrAAAAYAAJ.
- ^ Papp, John F. "Commodity Summary 2009: Chromium". United States Geological Survey. http://minerals.usgs.gov/minerals/pubs/commodity/chromium/mcs-2009-chrom.pdf. Retrieved 2009-03-17.
- ^ Fleischer, Michael (l982). "New Mineral Names". American Mineralogist 67: 854–860. http://www.minsocam.org/ammin/AM67/AM67_854.pdf.
- ^ Chromium (with location data), Mindat
- ^ Chromium from Udachnaya-Vostochnaya pipe, Daldyn, Daldyn-Alakit kimberlite field, Saha Republic (Sakha Republic; Yakutia), Eastern-Siberian Region, Russia, Mindat
- ^ Gonzalez, A. R.; Ndung'u, K; Flegal, AR (2005). "Natural Occurrence of Hexavalent Chromium in the Aromas Red Sands Aquifer, California". Environmental Science and Technology 39 (15): 5505–5511. doi:10.1021/es048835n. PMID 16124280.
- ^ a b Georges, Audi (2003). "The NUBASE Evaluation of Nuclear and Decay Properties". Nuclear Physics A (Atomic Mass Data Center) 729: 3–128. Bibcode 2003NuPhA.729....3A. doi:10.1016/j.nuclphysa.2003.11.001.
- ^ Birck, J. L.; Rotaru, M; Allegre, C (1999). "53Mn-53Cr evolution of the early solar system". Geochimica et Cosmochimica Acta 63 (23–24): 4111–4117. Bibcode 1999GeCoA..63.4111B. doi:10.1016/S0016-7037(99)00312-9.
- ^ Frei, Robert; Gaucher, Claudio; Poulton, Simon W.; Canfield, Don E. (2009). "Fluctuations in Precambrian atmospheric oxygenation recorded by chromium isotopes". Nature 461 (7261): 250–253. Bibcode 2009Natur.461..250F. doi:10.1038/nature08266. PMID 19741707.
- ^ a b Bailey, Ronald Albert Chemistry of the environment, Academic Press, 2002, ISBN 0120734613, p. 388
- ^ a b Schmidt, Max (1968). "VI. Nebengruppe" (in German). Anorganische Chemie II.. Wissenschaftsverlag. pp. 119–127.
- ^ Puigdomenech, Ignasi Hydra/Medusa Chemical Equilibrium Database and Plotting Software (2004) KTH Royal Institute of Technology
- ^ "US water has large amounts of likely carcinogen: study". Yahoo News. 2010-12-19. http://news.yahoo.com/s/afp/healthusenvironmentpollutionwater. Retrieved 2010-12-19.
- ^ Haxhillazi, Gentiana. "Preparation, Structure and Vibrational Spectroscopy of Tetraperoxo Complexes of CrV+, VV+, NbV+ and TaV+ (PhD thesis, University of Siegen, 2003)". University Siegen. http://deposit.ddb.de/cgi-bin/dokserv?idn=969592612&dok_var=d1&dok_ext=pdf&fi....
- ^ Nguyen, T. et al. (2005). "Synthesis of a Stable Compound with Fivefold Bonding Between Two Chromium(I) Centers". Science 310 (5749): 844–847. Bibcode 2005Sci...310..844N. doi:10.1126/science.1116789. PMID 16179432.
- ^ Cotterell, Maurice. (2004). The Terracotta Warriors: The Secret Codes of the Emperor's Army. Rochester: Bear and Company. ISBN 159143033X. Page 102.
- ^ a b c Guertin, Jacques; Jacobs, James Alan and Avakian, Cynthia P. (2005). Chromium (VI) Handbook. CRC Press. pp. 7–11. ISBN 9781566706087.
- ^ Vauquelin, Louis Nicolas (1798). "Memoir on a New Metallic Acid which exists in the Red Lead of Sibiria". Journal of Natural Philosophy, Chemistry, and the Art 3: 146. http://books.google.com/?id=6dgPAAAAQAAJ.
- ^ van der Krogt, Peter. "Chromium". http://elements.vanderkrogt.net/element.php?sym=Cr. Retrieved 2008-08-24.
- ^ a b c Dennis, J. K.; Such, T. E. (1993). "History of Chromium Plating". Nickel and Chromium Plating. Woodhead Publishing. pp. 9–12. ISBN 9781855730816.
- ^ a b c Papp, John F. "Mineral Yearbook 2002: Chromium". United States Geological Survey. http://minerals.usgs.gov/minerals/pubs/commodity/chromium/chrommyb02.pdf. Retrieved 2009-02-16.
- ^ a b c d Papp, John F; Lipin Bruce R. (2006). "Chromite". Industrial Minerals & Rocks: Commodities, Markets, and Uses (7th ed.). SME. ISBN 9780873352338. http://books.google.com/?id=zNicdkuulE4C&pg=PA309.
- ^ Bhadeshia, H. K. D. H.. "Nickel-Based Superalloys". University of Cambridge. http://www.msm.cam.ac.uk/phase-trans/2003/Superalloys/superalloys.html. Retrieved 2009-02-17.
- ^ Edwards, Joseph (1997). Coating and Surface Treatment Systems for Metals. Finishing Publications Ltd. and ASMy International. pp. 66–71. ISBN 0-904477-16-9.
- ^ Zhao, J. (2001). "Effects of chromate and chromate conversion coatings on corrosion of aluminum alloy 2024-T3". Surface and Coatings Technology 140 (1): 51–57. doi:10.1016/S0257-8972(01)01003-9. https://dspace03.it.ohio-state.edu/dspace/bitstream/1811/36519/1/55_FrankelG_EffectsChromateChromateConversion_2001_p51-57.pdf.
- ^ Sprague, J. A.; Smidt, F. A. (1994). ASM Handbook: Surface Engineering. ASM International. ISBN 9780871703842. http://books.google.com/?id=RGtsPjqUwy0C&pg=PA484. Retrieved 2009-02-17.
- ^ Worobec, Mary Devine; Hogue, Cheryl (1992). Toxic Substances Controls Guide: Federal Regulation of Chemicals in the Environment. Washington, D.C.: BNA Books. p. 13. ISBN 9780871797520. http://books.google.com/?id=CjWQ6_7AnI4C&pg=PA13.
- ^ Gettens, Rutherford John (1966). "Chrome yellow". Painting Materials: A Short Encyclopaedia. Courier Dover Publications. pp. 105–106. ISBN 9780486215976. http://books.google.com/?id=bdQVgKWl3f4C&pg=PA106.
- ^ Moss, S. C.; Newnham, R. E. (1964). "The chromium position in ruby". Zeitschrift fur Kristallographie 120 (4–5): 359–363. doi:10.1524/zkri.1964.120.4-5.359. http://rruff.geo.arizona.edu/doclib/zk/vol120/ZK120_359.pdf.
- ^ Gerd Anger et al. "Chromium Compounds" Ullmann's Encylopedia of Industrial Chemistry 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a07 067
- ^ a b Hingston, J. et al. (2001). "Leaching of chromated copper arsenate wood preservatives: a review". Environmental Pollution 111 (1): 53–66. doi:10.1016/S0269-7491(00)00030-0. PMID 11202715.
- ^ Brown, E. M. (1997). "A Conformational Study of Collagen as Affected by Tanning Procedures". Journal of the American Leather Chemists Association 92: 225–233.
- ^ Sreeram, K (2003). "Sustaining tanning process through conservation, recovery and better utilization of chromium". Resources, Conservation and Recycling 38 (3): 185–212. doi:10.1016/S0921-3449(02)00151-9.
- ^ Weckhuysen, Bert M. (1999). "Olefin polymerization over supported chromium oxide catalysts". Catalysis Today 51 (2): 215–221. doi:10.1016/S0920-5861(99)00046-2.
- ^ Twigg, M. V. E. (1989). "The Water-Gas Shift Reaction". Catalyst Handbook. ISBN 9780723408574. http://books.google.com/books?id=YlJRAAAAMAAJ.
- ^ Rhodes, C (1995). "Water-gas shift reaction: Finding the mechanistic boundary". Catalysis Today 23: 43–58. doi:10.1016/0920-5861(94)00135-O.
- ^ http://www.orgsyn.org/orgsyn/prep.asp?prep=cv2p0142
- ^ Mallinson, John C. (1993). "Chromium Dioxide". The foundations of magnetic recording. Academic Press. ISBN 9780124666269. http://books.google.com/?id=rNifWsBxnWkC&pg=PA32.
- ^ Garverick, Linda (1994). Corrosion in the Petrochemical Industry. ASM International. ISBN 9780871705051. http://books.google.com/?id=qTfNZZRO758C&pg=PA278.
- ^ K. R. Di Bona, S. Love, N. R. Rhodes, D. McAdory, S. H. Sinha, N. Kern, J. Kent, J. Strickland, A. Wilson, J. Beaird, J. Ramage, J. F. Rasco, J. B. Vincent "Chromium is not an essential trace element for mammals: effects of a "low-chromium" diet." Journal Biological Inorganic Chemistry, 2011, volume 16, p. 381-90. doi:10.1007/s00775-010-0734-y
- ^ Mertz, Walter (1 April 1993). "Chromium in Human Nutrition: A Review". Journal of Nutrition 123 (4): 626–33. PMID 8463863.
- ^ Moukarzel A (November 2009). "Chromium in parenteral nutrition: too little or too much?". Gastroenterology 137 (5 Suppl): S18–S28. doi:10.1053/j.gastro.2009.08.048. PMID 19874946.
- ^ "ToxFAQs: Chromium". Agency for Toxic Substances & Disease Registry, Centers for Disease Control and Prevention. February 2001. http://www.atsdr.cdc.gov/tfacts7.html. Retrieved 2007-10-02.
- ^ Heimbach, J.T.; Anderson, Richard A. (2005). "Chromium: Recent Studies Regarding Nutritional Roles and Safety". Nutrition Today 40 (4): 189––195. doi:10.1097/00017285-200507000-00013. http://journals.lww.com/nutritiontodayonline/Abstract/2005/07000/Chromium__Recent_Studies_Regarding_Nutritional.13.aspx.
- ^ Vincent,, John B . (2003). "The Potential Value and Toxicity of Chromium Picolinate as a Nutritional Supplement, Weight Loss Agent and Muscle Development Agent". Sports Medicine:Volume 33 (3): 213–230. doi:10.2165/00007256-200333030-00004. PMID 12656641.
- ^ Cronin, Joseph R. (2004). "The Chromium Controversy". Alternative and Complementary Therapies 10 (1): 39–42. doi:10.1089/107628004772830393.
- ^ Stearns, D. M.; W; P; W (1 December 1995). "Chromium(III) picolinate produces chromosome damage in Chinese hamster ovary cells". Federation of American Societies for Experimental Biology 9 (15): 1643–8. PMID 8529845.
- ^ Vincent, J. B. (2007). "Recent advances in the nutritional biochemistry of trivalent chromium". Proceedings of the Nutrition Society 63 (1): 41–47. doi:10.1079/PNS2003315. PMID 15070438.
- ^ Barceloux, Donald G.; Barceloux, Donald (1999). "Chromium". Clinical Toxicology 37 (2): 173–194. doi:10.1081/CLT-100102418. PMID 10382554.
- ^ a b Eastmond, David A.; MacGregor, JT; Slesinski, RS (2008). "Trivalent Chromium: Assessing the Genotoxic Risk of an Essential Trace Element and Widely Used Human and Animal Nutritional Supplement". Critical Reviews in Toxicology 38 (3): 173–190. doi:10.1080/10408440701845401. PMID 18324515.
- ^ a b Katz, Sidney A.; Salem, H (1992). "The toxicology of chromium with respect to its chemical speciation: A review". Journal of Applied Toxicology 13 (3): 217–224. doi:10.1002/jat.2550130314. PMID 8326093.
- ^ Dayan, A. D.; Paine, A. J. (2001). "Mechanisms of chromium toxicity, carcinogenicity and allergenicity: Review of the literature from 1985 to 2000". Human & Experimental Toxicology 20 (9): 439–451. doi:10.1191/096032701682693062. PMID 11776406.
- ^ Newman, D. (1890). "A case of adeno-carcinoma of the left inferior turbinated body, and perforation of thenasal septum, in the person of a worker in chrome pigments". Glasgow Medical Journal 33: 469–470.
- ^ Langard, Sverre (1990). "One Hundred Years of Chromium and Cancer: A Review of Epidemiological Evidence and Selected Case Reports". American Journal of Industrial Medicine 17 (2): 189–214. doi:10.1002/ajim.4700170205. PMID 2405656.
- ^ Cohen, M. D.; Kargacin, B.; Klein, C. B.; Costa, M. (1993). "Mechanisms of chromium carcinogenicity and toxicity". Critical reviews in toxicology 23 (3): 255–281. doi:10.3109/10408449309105012. PMID 8260068.
- ^ "Chrome Contact Allergy". DermNet NZ. http://dermnetnz.org/dermatitis/chrome-allergy.html.
- ^ Basketter, David; Horev, L.; Slodovnik, D.; Merimes, S.; Trattner, A.; Ingber, A. (2000). "Investigation of the threshold for allergic reactivity to chromium". Contact Dermatitis 44 (2): 70–74. doi:10.1034/j.1600-0536.2001.440202.x. PMID 11205406.
- ^ Baselt, Randall C. (2008). Disposition of Toxic Drugs and Chemicals in Man (8th ed.). Foster City: Biomedical Publications. pp. 305–307. ISBN 9780962652370.
External links
- ATSDR Case Studies in Environmental Medicine: Chromium Toxicity U.S. Department of Health and Human Services
- IARC Monograph "Chromium and Chromium compounds"
- It's Elemental – The Element Chromium
- National Pollutant Inventory – Chromium (III) compounds fact sheet
- The Merck Manual – Mineral Deficiency and Toxicity
- National Institute for Occupational Safety and Health – Chromium Page
- The periodic table of videos: Chromium
Periodic table H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Rf Db Sg Bh Hs Mt Ds Rg Cn Uut Uuq Uup Uuh Uus Uuo Alkali metals Alkaline earth metals Lanthanides Actinides Transition metals Other metals Metalloids Other nonmetals Halogens Noble gases Unknown chem. properties Large version Chromium compounds Categories:- Chemical elements
- Transition metals
- Chromium
- Dietary minerals
- Occupational safety and health
- Biology and pharmacology of chemical elements
- Greek loanwords
- 2 [CrO4]2- + 2 H+
Wikimedia Foundation. 2010.