- Sodium borohydride
-
Sodium borohydride 
 Sodium tetrahydridoborate(1–)Systematic nameSodium boranuide
Sodium tetrahydridoborate(1–)Systematic nameSodium boranuideIdentifiers CAS number 16940-66-2  , 15681-89-7 (2H4)
, 15681-89-7 (2H4)PubChem 4311764  , 23673181 (2H4)
, 23673181 (2H4)  , 23671303 (3H4)
, 23671303 (3H4) 
ChemSpider 26189  , 9052313 (2H4)
, 9052313 (2H4)  , 9312193 (3H4)
, 9312193 (3H4) 
EC number 241-004-4 UN number 1426 MeSH Sodium+borohydride ChEBI CHEBI:50985 
RTECS number ED3325000 Gmelin Reference 23167 Jmol-3D images Image 1 - [Na+].[BH4-]
Properties Molecular formula NaBH4 Molar mass 37.83 g/mol Appearance white crystals
hygroscopicDensity 1.0740 g/cm3 Melting point 400 °C[1]
Boiling point 500 °C (dec.)[1]
Solubility in water soluble, reacts with water Solubility soluble in liquid ammonia, amines, pyridine Hazards MSDS ICSC 1670 NFPA 704 Flash point 70 °C Autoignition
temperatureca. 220 °C LD50 160 mg/kg Related compounds Other anions Sodium cyanoborohydride
Sodium hydride
Sodium borate
BoraxOther cations Lithium borohydride Related compounds Lithium aluminium hydride  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Sodium borohydride, also known as sodium tetrahydridoborate, is an inorganic compound with the formula NaBH4. This white solid, usually encountered as a powder, is a versatile reducing agent that finds wide application in chemistry, both in the laboratory and on a technical scale. Large amounts are used for bleaching wood pulp. The compound is insoluble in ether, and soluble in glyme solvents, methanol and water, but reacts with the latter two in the absence of base.[2]
The compound was discovered in the 1940s by H. I. Schlesinger, who led a team that developed metal borohydrides for wartime applications.[3] Their work was classified and published only in 1953.
Contents
Physical properties
Sodium borohydride is an odorless white to gray-white microcrystalline powder which often forms lumps. It is soluble in water, without decomposition, however it reacts vigorously with acid solutions.
Structure
NaBH4 has three known polymorphs: α, β and γ. The stable phase at room temperature and pressure is α-NaBH4, which is cubic and adopts an NaCl-type structure, in the Fm3m space group. At a pressure of 6.3 GPa, the structure changes to the tetragonal β-NaBH4 (space group P421c) and at 8.9 GPa, the orthorhombic γ-NaBH4 (space group Pnma) becomes the most stable.[4][5][6]
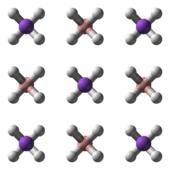
α-NaBH4 β-NaBH4 γ-NaBH4 Synthesis and handling
Sodium borohydride is prepared by two routes of industrial significance. In one method, based on the original work of Schlesinger, sodium hydride is treated with trimethyl borate at 250-270 °C:
- B(OCH3)3 + 4 NaH → NaBH4 + 3 NaOCH3
Alternatively, sodium borohydride is also produced by the action of NaH on powdered borosilicate glass. Millions of kilograms are produced annually, far exceeding the production levels of any other hydride reducing agent.[7][8]
NaBH4 can be recrystallized by dissolving in warm (50 °C) diglyme followed by cooling the solution.[9]
Reactivity
NaBH4 will reduce many organic carbonyls, depending on the precise conditions. Most typically, it is used in the laboratory for converting ketones and aldehydes to alcohols. It will reduce acyl chlorides, thiol esters and imines. However, unlike the powerful reducing agent lithium aluminium hydride, NaBH4 typically will not reduce esters, amides, or carboxylic acids.[2] At room temperature, the only acid derivatives it reduces are acyl chlorides, which have exceptionally high reactivity. With high temperature and concomitant high pressure, sodium borohydride can be forced to react with esters, for example.
Many other hydride reagents are more strongly reducing. These usually involve replacing hydride with alkyl groups, such as lithium triethylborohydride and L-Selectride (lithium tri-sec-butylborohydride), or replacing B with Al. Variations in the counterion also affect the reactivity of the borohydride.[10]
 Ball-and-stick model of Zr(BH4)4
Ball-and-stick model of Zr(BH4)4
Oxidation of NaBH4 with iodine in tetrahydrofuran gives the BH3-THF complex, which can reduce carboxylic acids. Likewise, the NaBH4-MeOH system, formed by the addition of methanol to sodium borohydride in refluxing THF, reduces esters to the corresponding alcohols, for instance, benzyl benzoate to benzyl alcohol.[11] Mixing water or an alcohol with the borohydride converts some of it into unstable hydride ester, which is more efficient at reduction, but the reductant will eventually decompose spontaneously to give hydrogen gas and borates. The same reaction can run also intramolecularly: an α-ketoester converts into a diol, since the alcohol produced will attack the borohydride to produce an ester of the borohydride, which then reduces the neighboring ester.[12]
Aqueous solutions of sodium borohydride are decomposed by catalytic amounts of cobalt(II) ions to yield sodium borate and hydrogen gas. Pellets of cobalt-doped sodium borohydride are commercially available for use in hydrogen generators, for applications where cylinders of hydrogen would be inconvenient.
BH4− is an excellent ligand for metal ions. Such borohydride complexes are often prepared by the action of NaBH4 (or the LiBH4) on the corresponding metal halide. One example is the titanocene derivative:[13]
- 2 (C5H5)2TiCl2 + 4 NaBH4 → 2 (C5H5)2TiBH4 + 4 NaCl + B2H6 + H2
Combustion
For more details on this topic, see Direct borohydride fuel cell.Sodium borohydride is less flammable and less volatile than gasoline, but more corrosive. It is relatively environmentally friendly because of the low toxicity of borates. The hydrogen is generated for a fuel cell by catalytic decomposition of the aqueous borohydride solution:
- NaBH4 + 2 H2O → NaBO2 + 4 H2 (ΔH < 0)
Applications
The principle application of sodium borohydride is the production of sodium dithionite, which is used as a bleaching agent for wood pulp. Sulfur dioxide reacts with the borohydride. In a related process, sodium dithionite is used in the dyeing industry. Sodium borohydride can also be used in oxymercuration reactions.
Production of pharmaceuticals
Sodium borohydride reduces aldehydes and ketones into alcohols. This reaction is used in the production of various antibiotics including chloramphenicol, dihydrostreptomycin, and thiophenicol. Various steroids and vitamin A are prepared using sodium borohydride in at least one step.
Safety
Sodium borohydride is a source of basic borate salts which can be corrosive, and hydrogen or diborane, which are both flammable. Spontaneous ignition can result from solution of sodium borohydride in dimethylformamide.
See also
References
- ^ a b MSDS data (carl roth)
- ^ a b Banfi, L.; Narisano, E.; Riva, R.; Stiasni, N.; Hiersemann, M. “Sodium Borohydride” in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. doi:10.1002/047084289X.rs052.
- ^ Schlesinger, H. I.; Brown, H. C.; Abraham, B.; Bond, A. C.; Davidson, N.; Finholt, A. E.; Gilbreath, J. R.; Hoekstra, H.; Horvitz, L.; Hyde, E. K.; Katz, J. J.; Knight, J.; Lad, R. A.; Mayfield, D. L.; Rapp, L.; Ritter, D. M.; Schwartz, A. M.; Sheft, I.; Tuck, L. D.; Walker, A. O. (1953). "New developments in the chemistry of diborane and the borohydrides. General summary". J. Am. Chem. Soc. 75: 186–90. doi:10.1021/ja01097a049.
- ^ R. S. Kumar, A. L. Cornelius (2005). "Structural transitions in NaBH[sub 4] under pressure". Appl. Phys. Lett. 87 (26): 261916. doi:10.1063/1.2158505.
- ^ Y. Filinchuk, A. V. Talyzin, D. Chernyshov, V. Dmitriev (2007). "High-pressure phase of NaBH4: Crystal structure from synchrotron powder diffraction data". Phys. Rev. B 76 (9): 092104. doi:10.1103/PhysRevB.76.092104.
- ^ E. Kim, R. Kumar, P. F. Weck, A. L. Cornelius, M. Nicol, S. C. Vogel, J. Zhang, M. Hartl, A. C. Stowe, L. Daemen, Y. Zhao (2007). "Pressure-driven phase transitions in NaBH4: theory and experiments". J. Phys. Chem. B 111 (50): 13873–13876. doi:10.1021/jp709840w. PMID 18031032.
- ^ Peter Rittmeyer, Ulrich Wietelmann “Hydrides” in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a13_199
- ^ Schubert, F.; Lang, K.; Burger, A. “Alkali metal borohydrides” (Bayer), 1960. German patent DE 1088930 19600915 (ChemAbs: 55:120851). Supplement to . to Ger. 1,067,005 (CA 55, 11778i). From the abstract: “Alkali metal borosilicates are treated with alkali metal hydrides in approx. 1:1 ratio at >100 °C with or without H pressure”.
- ^ Brown, H. C. “Organic Syntheses via Boranes” John Wiley & Sons, Inc. New York: 1975. ISBN 0-471-11280-1. page 260-1.
- ^ Seyden-Penne, J. "Reductions by the Alumino- and Borohydrides in Organic Synthesis"; VCH–Lavoisier: Paris, 1991.
- ^ da Costa, Jorge C.S.; Karla C. Pais, Elisa L. Fernandes, Pedro S. M. de Oliveira, Jorge S. Mendonça, Marcus V. N. de Souza, Mônica A. Peralta, and Thatyana R.A. Vasconcelos (2006). "Simple reduction of ethyl, isopropyl and benzyl aromatic esters to alcohols using sodium borohydride-methanol system" (PDF). Arkivoc: 128–133. http://www.arkat-usa.org/ark/journal/2006/I01_General/1523/05-1523A%20as%20published%20mainmanuscript.pdf. Retrieved 2006-08-29.
- ^ V. Dalla, J. P. Catteau and P. Pale. Mechanistic rationale for the NaBH4 reduction of α-keto esters. Tetrahedron Letters, Volume 40, Issue 28, 9 July 1999, Pages 5193-5196.
- ^ C. R. Lucas, “Bis(5-Cyclopentadienyl) [Tetrahydroborato(1-)]Titanium” Inorganic Syntheses, 1977, Volume 17, p.93. doi:10.1002/9780470132487.ch27
External links
- National Pollutant Inventory - Boron and compounds
- MSDS for Sodium Borohydride
- Materials & Energy Research Institute Tokyo, Ltd.
- Chemo- and stereoselectivity using Borohydride reagents
- Sodium Borohydride Digest, Rohm & Haas
Sodium compounds NaAlO2 · NaBH3(CN) · NaBH4 · NaBr · NaBrO3 · NaCH3COO · NaCN · NaC6H5CO2 · NaC6H4(OH)CO2 · NaCl · NaClO · NaClO2 · NaClO3 · NaClO4 · NaF · NaH · NaHCO3 · NaHSO3 · NaHSO4 · NaI · NaIO3 · NaIO4 · NaMnO4 · NaNH2 · NaNO2 · NaNO3 · NaN3 · NaOH · NaO2 · NaPO2H2 · NaReO4 · NaSCN · NaSH · NaTcO4 · NaVO3 · Na2CO3 · Na2C2O4 · Na2CrO4 · Na2Cr2O7 · Na2MnO4 · Na2MoO4 · Na2O · Na2O2 · Na2O(UO3)2 · Na2S · Na2SO3 · Na2SO4 · Na2S2O3 · Na2S2O4 · Na2S2O5 · Na2S2O6 · Na2S2O7 · Na2S2O8 · Na2Se · Na2SeO3 · Na2SeO4 · Na2SiO3 · Na2Te · Na2TeO3 · Na2Ti3O7 · Na2U2O7 · NaWO4 · Na2Zn(OH)4 · Na3N · Na3P · Na3VO4 · Na4Fe(CN)6 · Na5P3O10 · NaBiO3
Categories:- Sodium compounds
- Borohydrides
- Reducing agents
Wikimedia Foundation. 2010.

