- Transcranial magnetic stimulation
-
For other uses, see TMS (disambiguation).
Transcranial magnetic stimulation Intervention 
rTMS in a rodent.MeSH D050781 Transcranial magnetic stimulation (TMS) is a noninvasive method to cause depolarization or hyperpolarization in the neurons of the brain. TMS uses electromagnetic induction to induce weak electric currents using a rapidly changing magnetic field; this can cause activity in specific or general parts of the brain with minimal discomfort, allowing the functioning and interconnections of the brain to be studied. A variant of TMS, repetitive transcranial magnetic stimulation (rTMS), has been tested as a treatment tool for various neurological and psychiatric disorders including migraines, strokes, Parkinson's disease, dystonia, tinnitus, depression and auditory hallucinations.
Contents
Background
The principle of inductive brain stimulation with eddy currents has been noted since the 20th century. The first successful TMS study was performed in 1985 by Anthony Barker and his colleagues in Sheffield, England.[1] Its earliest application demonstrated conduction of nerve impulses from the motor cortex to the spinal cord, stimulating muscle contractions. The use of magnets rather than a direct electric current to the brain reduced the discomfort of the procedure and research and allowed mapping of the cerebral cortex and its connections.
Effects on the brain
The exact details of how TMS functions are still being explored. The effects of TMS can be divided into two types depending on the mode of stimulation:
- Single or paired pulse TMS causes neurons in the neocortex under the site of stimulation to depolarize and discharge an action potential. If used in the primary motor cortex, it produces muscle activity referred to as a motor evoked potential (MEP) which can be recorded on electromyography. If used on the occipital cortex, 'phosphenes' (flashes of light) might be perceived by the subject. In most other areas of the cortex, the participant does not consciously experience any effect, but his or her behaviour may be slightly altered (e.g. slower reaction time on a cognitive task), or changes in brain activity may be detected using sensing equipment.[2]
- Repetitive TMS produces longer-lasting effects which persist past the initial period of stimulation. rTMS can increase or decrease the excitability of the corticospinal tract depending on the intensity of stimulation, coil orientation and frequency. The mechanism of these effects is not clear although it is widely believed to reflect changes in synaptic efficacy akin to long-term potentiation (LTP) and long-term depression (LTD).[3]
Risks
Although TMS is often regarded as safe, the greatest acute risk of TMS is the rare occurrence of induced seizures and syncope.[4] More than 16 cases of TMS-related seizure have been reported in the literature, with at least seven reported before the publication of safety guidelines in 1998,[5] and more than nine reported afterwards. The seizures have been associated with single-pulse and rTMS. Reports have stated that in at least some cases, predisposing factors (medication, brain lesions or genetic susceptibility) may have contributed to the seizure. A review of nine seizures associated with rTMS that had been reported after 1998 stated that four seizures were within the safety parameters, four were outside of those parameters, and one had occurred in a healthy volunteer with no predisposing factors. A 2009 international consensus statement on TMS that contained this review concluded that based on the number of studies, subjects and patients involved with TMS research, the risk of seizure with rTMS is considered very low.[4]
Besides seizures, other risks include fainting, minor pains such as headache or local discomfort, minor cognitive changes and psychiatric symptoms (particularly a low risk of mania in depressed patients).[4] Though other side effects are thought to be possibly associated with TMS (alterations to the endocrine system, altered neurotransmitter and immune system activity) they are considered investigational and lacking substantive proof.[4]
Other adverse effects of TMS are:
- Discomfort or pain from the stimulation of the scalp and associated nerves and muscles on the overlying skin;[6] this is more common with rTMS than single pulse TMS,[5]
- Rapid deformation of the TMS coil produces a loud clicking sound which increases with the stimulator intensity that can affect hearing with sufficient exposure, particularly relevant for rTMS (hearing protection may be used to prevent this),[5]
- rTMS in the presence of incompatible EEG electrodes can result in electrode heating and, in severe cases, skin burns.[7] Non-metallic electrodes are used if concurrent EEG data is required.
Clinical uses
The uses of TMS and rTMS can be divided into diagnostic and therapeutic uses.
Diagnosis
TMS can be used clinically to measure activity and function of specific brain circuits in humans. The most robust and widely-accepted use is in measuring the connection between the primary motor cortex and a muscle to evaluate damage from strokes, spinal cord injuries, multiple sclerosis and motor neuron disease.[8][9][10] TMS has been suggested as a means of assessing short-interval intracortical inhibition (SICI) which measures the internal pathways of the motor cortex but this use has not yet been validated.[11]
Therapy
Studies of the use of TMS and rTMS to treat neurological and psychiatric conditions have shown only modest effects with little confirmation of results.[12] However, publications reporting the results of reviews and statistical meta-analyses of earlier investigations have stated that rTMS appeared to be effective in the treatment of certain types of major depression under certain specific conditions.[12][13][14][15][16][17] rTMS devices are marketed for the treatment of such disorders in Canada, Australia, New Zealand, the European Union, Israel and the United States.[14][18]
A recent meta-analysis of 34 studies comparing rTMS to sham treatment showed an effect size of 0.55 (p<.001).[19] This is comparable to commonly reported effect sizes of pharmacotherapeutic strategies for treatment of depression in the range of 0.17-0.46.[19] However, that same meta-analysis found that rTMS was significantly worse than electroconvulsive therapy (effect size -0.47), although side effects were significantly better with rTMS. An analysis of one of the studies included in the meta-analysis showed that one extra remission from depression occurs for every 3 patients given electroconvulsive therapy rather than rTMS (number needed to treat 2.36).[20]
There is evidence that rTMS can temporarily reduce chronic pain and change pain-related brain and nerve activity, and TMS has been used to predict the success of surgically implanted electrical brain stimulation for the treatment of pain.[21]
Other areas of research include the rehabilitation of aphasia and motor disability after stroke,[4][9][10][22] tinnitus,[23] Parkinson's disease[24][25] and the negative symptoms of schizophrenia.[26] TMS has failed to show effectiveness for the treatment of brain death, coma, and other persistent vegetative states.[27]
It is difficult to establish a convincing form of "sham" TMS to test for placebo effects during controlled trials in conscious individuals, due to the neck pain, headache and twitching in the scalp or upper face associated with the intervention.[4] "Sham" TMS manipulations can affect cerebral glucose metabolism and MEPs, which may confound results.[14] This problem is exacerbated when using subjective measures of improvement. Depending on the research question asked and the experimental design, matching this discomfort to distinguish true effects from placebo can be an important and challenging issue.[4]
A recent multicenter trial of rTMS in depression used a "sham" placebo treatment that appeared to mimic the sound and scalp stimulation associated with active TMS treatment. The investigators concluded: "Although the treatment effect was statistically significant on a clinically meaningful variable (remission), the overall number of remitters and responders was less than one would like with a treatment that requires daily intervention for 3 weeks or more, even with a benign adverse effect profile".[28] However, a review of the trial's report has questioned the adequacy of the placebo, noting that treaters were able to guess whether patients were receiving treatment with active or sham TMS, better than chance.[29]
FDA actions and responses
FDA actions
In January 2007 an advisory panel of the United States Food and Drug Administration (FDA) did not recommend clearance for marketing of an rTMS device, stating that the device appeared to be reasonably safe but had failed to demonstrate efficacy in a study of people with major depression who had not benefitted from prior adequate treatment with oral antidepressants during their current major depressive episode.[30] The panel agreed that "unblinding was greater in the active group, and considering the magnitude of the effect size, it may have influenced the study results."[30] However, the FDA determined in December 2008 that the rTMS device was sufficiently similar to existing devices that did not require a premarket approval application and allowed the device to be marketed in accordance with Section 510(k) of the Federal Food, Drug, and Cosmetic Act for "the treatment of Major Depressive Disorder in adult patients who have failed to achieve satisfactory improvement from one prior antidepressant medication at or above the minimal effective dose and duration in the current episode".[18] The user manual for the device warns that effectiveness has not been established in patients with major depressive disorder who have failed to achieve satisfactory improvement from zero and from two or more antidepressant medications in the current episode and that the device has not been studied in patients who have had no prior antidepressant medication.[31]
In July 2011 the FDA published a final rule in the Federal Register that classified the rTMS system into class II (special controls) (see: Medical device#Classification) "in order to provide a reasonable assurance of safety and effectiveness of these devices". The rule identified the rTMS system as "an external device that delivers transcranial pulsed magnetic fields of sufficient magnitude to induce neural action potentials in the prefrontal cortex to treat the symptoms of major depressive disorder without inducing seizure in patients who have failed at least one antidepressant medication and are currently not on any antidepressant therapy".[32] An FDA guidance document issued in conjunction with the final rule describes the special controls that support the classification of the rTMS system into Class II.[33]
Response to FDA decision
Soon after the FDA cleared the device, several members of Public Citizen stated in a letter to the editor of the medical journal Neuropsychopharmacology that the FDA seemed to have based its decision on a post-hoc analysis that did not establish the effectiveness of rTMS for the treatment of depression. The writers of the letter expressed their concern that patients would be diverted from therapies such as antidepressant medications that have an established history of effectiveness.[34]
Technical information
 TMS - Butterfly Coils
TMS - Butterfly Coils
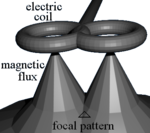
TMS uses electromagnetic induction to generate an electric current across the scalp and skull without physical contact. A plastic-enclosed coil of wire is held next to the skull and when activated, produces a magnetic field oriented orthogonally to the plane of the coil. The magnetic field passes unimpeded through the skin and skull, inducing an oppositely directed current in the brain that activates nearby nerve cells in much the same way as currents applied directly to the cortical surface.[35]
The path of this current is difficult to model because the brain is irregularly shaped and electricity and magnetism are not conducted uniformly throughout its tissues. The magnetic field is about the same strength as an MRI, and the pulse generally reaches no more than 5 centimeters into the brain.[36]
Coil types
The design of transcranial magnetic stimulation coils used in either treatment or diagnostic/experimental studies may differ in a variety of ways. These differences should be considered in the interpretation of any study result, and the type of coil used should be specified in the study methods for any published reports.
The most important considerations include:
- the type of material used to construct the core of the coil
- the geometry of the coil configuration
- the biophysical characteristics of the pulse produced by the coil.
With regard to coil composition, the core material may be either a magnetically inert substrate (i.e., the so-called ‘air-core’coil design), or possess a solid, ferromagnetically active material (ie, the so-called ‘solid-core’ design). Solid core coil design result in a more efficient transfer of electrical energy into a magnetic field, with a substantially reduced amount of energy dissipated as heat, and so can be operated under more aggressive duty cycles often mandated in therapeutic protocols, without treatment interruption due to heat accumulation, or the use of an accessory method of cooling the coil during operation. Varying the geometric shape of the coil itself may also result in variations in the focality, shape, and depth of cortical penetration of the magnetic field. Differences in the coil substance as well as the electronic operation of the power supply to the coil may also result in variations in the biophysical characteristics of the resulting magnetic pulse (e.g., width or duration of the magnetic field pulse). All of these features should be considered when comparing results obtained from different studies, with respect to both safety and efficacy.[37]
A number of different types of coils exist, each of which produce different magnetic field patterns. Some examples:
- round coil: the original type of TMS coil
- figure-eight coil (i.e. butterfly coil): results in a more focal pattern of activation
- double-cone coil: conforms to shape of head, useful for deeper stimulation
- four-leaf coil: for focal stimulation of peripheral nerves[38]
Design variations in the shape of the TMS coils allow much deeper penetration of the brain than the standard depth of 1.5 cm. Circular, H-shaped, double cone coils and other experimental variations can induce excitation or inhibition of neurons deeper in the brain including activation of motor neurons for the cerebellum, legs and pelvic floor. Though able to penetrate deeper in the brain, they are less able to produced a focused, localized response and are relatively non-focal.[4]
See also
- Cranial electrotherapy stimulation
- Transcranial direct current stimulation
- Electroconvulsive therapy
- God helmet
- Neuronetics
References
- ^ Barker AT, Jalinous R, Freeston IL. (May 1985). "Non-invasive magnetic stimulation of human motor cortex". The Lancet 1 (8437): 1106–1107. doi:10.1016/S0140-6736(85)92413-4. PMID 2860322.
- ^ Pascual-Leone A; Davey N; Rothwell J; Wassermann EM; Puri BK (2002). Handbook of Transcranial Magnetic Stimulation. Hodder Arnold. ISBN 0340720093.
- ^ Fitzgerald PB; Fountain S; Daskalakis J (December 2006). "A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition". Clinical Neurophysiology 117 (12): 2584–96. doi:10.1016/j.clinph.2006.06.712. PMID 16890483.
- ^ a b c d e f g h Rossi, S; et al. (2009). "The Safety of TMS Consensus Group, Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research" (pdf). Clinical Neurophysiology 120 (12): 2008–2039. doi:10.1016/j.clinph.2009.08.016. PMID 19833552. http://www.aipass.org/files/TMS_Safety,%20ethical%20considerations,%20and%20application%20guidelines.pdf.
- ^ a b c Wassermann EM (1998). "Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996" (pdf). Electroencephalography and clinical Neurophysiology 108 (1): 1–16. doi:10.1016/S0168-5597(97)00096-8. PMID 9474057. http://www.icts.uci.edu/neuroimaging/Wassermann_rTMS_Safety1998.pdf.
- ^ "Transcranial Magnetic Stimulation (TMS)". National Alliance on Mental Illness. http://www.nami.org/Content/ContentGroups/Helpline1/Transcranial_Magnetic_Stimulation_(rTMS).htm. Retrieved 2008-12-15.
- ^ Roth BJ; Pascual-Leone A; Cohen LG; Hallett M (1992). "The heating of metal electrodes during rapid-rate magnetic stimulation: A possible safety hazard". Electroenceph. Clin. Neurophysiol. 85 (2): 116–123. doi:10.1016/0168-5597(92)90077-O. PMID 1373364.
- ^ Rossini, P.; Rossi, S. (2007). "Transcranial magnetic stimulation: diagnostic, therapeutic, and research potential". Neurology 68 (7): 484–488. doi:10.1212/01.wnl.0000250268.13789.b2. PMID 17296913.
- ^ a b Dimyan, MA; Cohen, L (2010). "Contribution of transcranial magnetic stimulation to the understanding of mechanisms of functional recovery after stroke". Neurorehabilitation and Neural Repair 24 (2): 125–135. doi:10.1177/1545968309345270. PMC 2945387. PMID 19767591. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2945387.
- ^ a b Nowak, D.; Bösl, K.; Podubeckà, J.; Carey, J. (2010). "Noninvasive brain stimulation and motor recovery after stroke". Restorative Neurology and Neuroscience 28 (4): 531–544. doi:10.3233/RNN-2010-0552. PMID 20714076.
- ^ Kujirai T, Caramia MD, Rothwell JC, et al. (November 1993). "Corticocortical inhibition in human motor cortex". J. Physiol. (Lond.) 471: 501–19. PMC 1143973. PMID 8120818. http://www.jphysiol.org/cgi/pmidlookup?view=long&pmid=8120818.
- ^ a b Slotema, CW; Blom, JD; Hoek, HW; Sommer, IE (July 2010). "Should we expand the toolbox of psychiatric treatment methods to include Repetitive Transcranial Magnetic Stimulation (rTMS)? a meta-analysis of the efficacy of rTMS in psychiatric disorders". Journal of Clinical Psychiatry (Physicians Postgraduate Press, Inc.) 71 (7): 873–884. doi:10.4088/JCP.08m04872gre. PMID 20361902.
- ^ RTI International-University of North Carolina (RTI-UNC) Evidence-based Practice Center, Research Triangle Park, North Carolina (September 2011). "Nonpharmacologic Interventions for Treatment-Resistant Depression in Adults". Effective Health Care Program: Comparative Effectiveness Review: Number 33. Rockville, Maryland: Agency for Healthcare Research and Quality, United States Department of Health and Human Services. http://www.effectivehealthcare.ahrq.gov/ehc/products/76/792/TRD_Final-Report_20110926.pdf. Retrieved 2011-09-26.
- ^ a b c Marangell, L. B.; Martinez, M.; Jurdi, R. A.; Zboyan, H. (2007). "Neurostimulation therapies in depression: a review of new modalities". Acta Psychiatrica Scandinavica 116 (3): 174. doi:10.1111/j.1600-0447.2007.01033.x. PMID 17655558.
- ^ Gross, M.; Nakamura, L.; Pascual-Leone, A.; Fregni, F. (2007). "Has repetitive transcranial magnetic stimulation (rTMS) treatment for depression improved? A systematic review and meta-analysis comparing the recent vs. the earlier rTMS studies". Acta Psychiatrica Scandinavica 116 (3): 165–173. doi:10.1111/j.1600-0447.2007.01049.x. PMID 17655557.
- ^ Lam, RW; Chan, P; Wilkins-Ho, M; Yatham, LV (September 2008). "Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and metaanalysis". Canadian Journal of Psychiatry 53 (9): 621–631. PMID 18801225.
- ^ Schutter, D. (2008). "Antidepressant efficacy of high-frequency transcranial magnetic stimulation over the left dorsolateral prefrontal cortex in double-blind sham-controlled designs: a meta-analysis". Psychological Medicine 39 (1): 65–75. doi:10.1017/S0033291708003462. PMID 18447962.
- ^ a b Melkerson, MN (2008-12-16). "Special Premarket 510(k) Notification for NeuroStar TMS Therapy System for Major Depressive Disorder" (pdf). FDA. http://www.accessdata.fda.gov/cdrh_docs/pdf8/K083538.pdf. Retrieved 2010-07-16.
- ^ a b Slotema et al. Should we expand the toolbox of psychiatric treatment methods to include repetitive transcranial magnetic stimulation? A meta-analysis of the efficacy of rTMS in Psychiatric Disorders. J. Clin Psychiatry, 71:7, July 2010
- ^ Eranti et al. A randomized, controlled trial with 6 mo follow-up of rTMS and ECT for severe depression. Am J. Psychiatry, 164: 1, Jan 2007
- ^ Rosen AC, Ramkumar M, Nguyen T, Hoeft F (February 2009). "Noninvasive transcranial brain stimulation and pain". Curr Pain Headache Rep 13 (1): 12–7. doi:10.1007/s11916-009-0004-2. PMC 2697608. PMID 19126365. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2697608.
- ^ Martin, P. I.; Naeser, M. A.; Ho, M.; Treglia, E.; Kaplan, E.; Baker, E. H.; Pascual-Leone, A. (2009). "Research with Transcranial Magnetic Stimulation in the Treatment of Aphasia". Current Neurology and Neuroscience Reports 9 (6): 451–458. doi:10.1007/s11910-009-0067-9. PMC 2887285. PMID 19818232. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2887285.
- ^ Kleinjung, T; Vielsmeier, V; Landgrebe, M; Hajak, G; Langguth, B (2008). "Transcranial magnetic stimulation: a new diagnostic and therapeutic tool for tinnitus patients". The international tinnitus journal 14 (2): 112–8. PMID 19205161.
- ^ Lefaucheur, J. P. (2009). "Treatment of Parkinson’s disease by cortical stimulation". Expert Review of Neurotherapeutics 9 (12): 1755–1771. doi:10.1586/ern.09.132. PMID 19951135.
- ^ Arias-Carrión, O. (2008). "Basic mechanisms of rTMS: Implications in Parkinson's disease". International Archives of Medicine 1 (1): 2. doi:10.1186/1755-7682-1-2. PMC 2375865. PMID 18471317. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2375865.
- ^ Dlabač-De Lange, J. J.; Knegtering, R.; Aleman, A. � (2010). "Repetitive Transcranial Magnetic Stimulation for Negative Symptoms of Schizophrenia". The Journal of Clinical Psychiatry 71 (4): 411. doi:10.4088/JCP.08r04808yel. PMID 20361909.
- ^ Lapitska, N; Gosseries, O; Delvaux, V; Overgaard, M; Nielsen, F; Maertens De Noordhout, A; Moonen, G; Laureys, S (2009). "Transcranial magnetic stimulation in disorders of consciousness". Reviews in the neurosciences 20 (3-4): 235–50. PMID 20157993.
- ^ George, MS; Lisanby, SH; Avery, D; McDonald, WM; Durkalski, V; Pavlicova, M; Anderson, B; Nahas, Z et al. (2010). "Daily Left Prefrontal Transcranial Magnetic Stimulation Therapy for Major Depressive Disorder: A Sham-Controlled Randomized Trial". Archives of General Psychiatry 67 (5): 507–516. doi:10.1001/archgenpsychiatry.2010.46. PMID 20439832.
- ^ Mattes, Jeffrey A (2010-06-26). "TMS: Does it Really Work". American Medical Association. http://archpsyc.ama-assn.org/cgi/eletters/67/5/507#13743. Retrieved 2010-10-04.
- ^ a b Scudiero, JL (2007-01-26). "Brief Summary From the Neurological Devices Panel Meeting - January 26, 2007". FDA. http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/NeurologicalDevicesPanel/ucm124779.htm. Retrieved 2010-07-14. "The Panel’s consensus was that the efficacy was not established; some stated that the device’s effectiveness was “small,” “borderline,” “marginal” and “of questionable clinical significance.”"
- ^ NeuroStar TMS Therapy System User Manual. 1. Neuronetics, Inc.. pp. 1–5. http://www.neuronetics.com/pdf/Prescribing%20Information.pdf. Retrieved 2010-09-13.
- ^ Stade, NK, Deputy Director for Policy, Center for Devices and Radiological Health, Food and Drug Administration, United States Department of Health and Human Services (2011-07-26). "Medical Devices; Neurological Devices; Classification of Repetitive Transcranial Magnetic Stimulation System: Final rule". Federal Register (United States Government Printing Office) 76 (143): 44489–44491. http://www.gpo.gov/fdsys/pkg/FR-2011-07-26/pdf/2011-18806.pdf. Retrieved 2011-08-11.
- ^ U.S. Department of Health and Human Services: Food and Drug Administration: Center for Devices and Radiological Health: Office of Device Evaluation: Division of Ophthalmic, Neurological and Ear, Nose and Throat Devices: Neurodiagnostic and Neurotherapeutic Devices Branch (2011-07-26). "Guidance for Industry and FDA Staff - Class II Special Controls Guidance Document: Repetitive Transcranial Magnetic Stimulation (rTMS) Systems". U.S. Food and Drug Administration. http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm265269.htm. Retrieved 2011-08-10.
- ^ Hines, JZ; Lurie, P; Wolfe SM, Sidney M (2009). "Reply to Lisanby et al.: Post hoc analysis does not establish effectiveness of rTMS for depression" (pdf). Neuropsychopharmacology 34 (8): 2053–2054. doi:10.1038/npp.2009.22. PMID 19528946. http://www.nature.com/npp/journal/v34/n8/pdf/npp200922a.pdf.
- ^ John T. Cacioppo, Louis G. Tassinary, Gary G. Berntson, ed (2007). Handbook of psychophysiology (3rd ed.). New York, NY: Cambridge Univ. Press. pp. 121. ISBN 0521844711.
- ^ "Brain Stimulation Therapies". National Institute of Mental Health. 2009-11-17. http://www.nimh.nih.gov/health/topics/brain-stimulation-therapies/brain-stimulation-therapies.shtml. Retrieved 2010-07-14.
- ^ Riehl M (2008). "TMS Stimulator Design". In Wassermann EM, Epstein CM, Ziemann U, Walsh V, Paus T, Lisanby SH. Oxford Handbook of Transcranial Stimulation. Oxford: Oxford University Press. pp. 13–23, 25–32. ISBN 0198568924.
- ^ Roth BJ, Maccabee PJ, Eberle L, Amassian VE, Hallett M, Cadwell J, Anselmi GD, Tatarian GT (1994). "In-vitro evaluation of a four-leaf coil design for magnetic stimulation of peripheral nerve.". Electroenceph. Clin. Neurophysiol. 93 (1): 68–74. doi:10.1016/0168-5597(94)90093-0. PMID 7511524.
Further reading
- Wassermann, E.M; Epstein, C.M.; Ziemann, U.; Walsh, V.; Paus, T.; Lisanby, S.H. (2008). Oxford Handbook of Transcranial Stimulation (Oxford Handbooks). Oxford University Press, USA. ISBN 0-19-856892-4. http://books.google.com/?id=YeKleGrKwC4C&printsec=frontcover#v=onepage&q.
External links
Categories:- Neurophysiology
- Neuropsychology
- Neurotechnology
- Magnetic devices
- Electrotherapy
- Treatment of bipolar disorder
- Physical psychiatric treatments
Wikimedia Foundation. 2010.