- Bis(triphenylphosphine)iminium chloride
-
Bis(triphenylphosphine)iminium chloride  μ-nitrido-Bis(triphenylphosphorus) chlorideOther namesPPN chloride
μ-nitrido-Bis(triphenylphosphorus) chlorideOther namesPPN chloride
Bis(triphenylphosphine)iminium chloride
Bis(triphenylphosphoranylidene)iminium chloride
Bis(triphenylphosphoranylidene)ammonium chloride
Hexaphenyldiphosphazenium chloride
SelectophoreIdentifiers CAS number 21050-13-5 PubChem 3036656 ChemSpider 2300634 
Jmol-3D images Image 1 - [Cl-].N([P+](c1ccccc1)(c2ccccc2)c3ccccc3)=P(c4ccccc4)(c5ccccc5)c6ccccc6
- InChI=1S/C36H30NP2.ClH/c1-7-19-31(20-8-1)38(32-21-9-2-10-22-32,33-23-11-3-12-24-33)37-39(34-25-13-4-14-26-34,35-27-15-5-16-28-35)36-29-17-6-18-30-36;/h1-30H;1H/q+1;/p-1

Key: LVRCYPYRKNAAMX-UHFFFAOYSA-M
InChI=1/C36H30NP2.ClH/c1-7-19-31(20-8-1)38(32-21-9-2-10-22-32,33-23-11-3-12-24-33)37-39(34-25-13-4-14-26-34,35-27-15-5-16-28-35)36-29-17-6-18-30-36;/h1-30H;1H/q+1;/p-1
Key: LVRCYPYRKNAAMX-REWHXWOFAO
Properties Molecular formula C36H30ClNP2 Molar mass 574.03 g/mol Appearance colourless solid Density ?? g/cm3, solid Melting point 260-2 °C
Solubility in water moderate Hazards R-phrases 36/37/38 S-phrases 26-36 Related compounds Related compounds Tetraphenylarsonium chloride
Tetrabutylammonium chloride
tetrabutylammonium chloride (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Bis(triphenylphosphine)iminium chloride is the chemical compound with the formula [(C6H5)3P)2N]Cl, often written [(Ph3P)2N]Cl and abbreviated PPNCl. This colorless salt is a source of the PPN+ cation, which is used to isolate reactive anions. PPN+ is a phosphazene.
Synthesis and structure
PPNCl is prepared in two steps from triphenylphosphine:[1]
- Ph3P + Cl2 → Ph3PCl2
This triphenylphosphine dichloride is related to phosphorus pentachloride. Treatment of this species with hydroxylamine in the presence of Ph3P results in replacement of the P-Cl bonds by P=N bonds:
- 2 Ph3PCl2 + NH2OH·HCl + Ph3P → {[Ph3P]2N}Cl + 4HCl + Ph3PO
In PPN+ salts, P-N bond lengths are equivalent, 1.58 Å. The cation is bent at the central nitrogen, and has no inversion centre. Its connectivity is indicated by Ph3P=N=PPh3+.
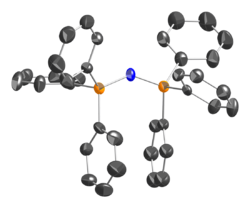
Applications
In the laboratory, PPN chloride is the main precursor to PPN+ salts. Using salt metathesis reactions, nitrite, azide, and other small inorganic anions can be obtained with PPN+ cations. The resulting salts PPNNO2, PPNN3 etc are soluble in polar organic solvents.
PPN+ forms crystalline salts with a range of anions that are otherwise difficult to crystallize. Its effectiveness is partially attributable to its rigidity, reflecting the presence of six phenyl rings. Often PPN+ forms salts that are more air-stable than salts with smaller cations such as those containing quaternary ammonium or alkali metal cations. This effect is attributed to the steric shielding provided by this voluminous cation. Illustrative PPN+ salts of reactive anions include PPN[HFe(CO)4], PPN[Co(CO)4], and PPN[Fe(CO)3NO]. The role of ion pairing in chemical reactions is often clarified by examination of the related salt derived from PPN+.
References
- ^ J. K. Ruff; W. J. Schlientz; R. E. Dessy; J. M. Malm; G. R. Dobson; M. N. Memering (1974). "μ-nitrido-Bis(triphenylphosphorus)(1+ (“PPN”) Salts with Metal Carbonyl Anions". Inorg. Synth. 15: 84–90. doi:10.1002/9780470132463.ch19.
Categories:- Phosphorus compounds
Wikimedia Foundation. 2010.
