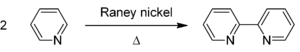- 2,2'-Bipyridine
-
2,2'-Bipyridine  2,2'-BipyridineOther namesBipyridyl
2,2'-BipyridineOther namesBipyridyl
Dipyridyl
Bipy
Bpy
DipyIdentifiers CAS number 366-18-7 ChemSpider 13867714 
UNII 551W113ZEP ChEBI CHEBI:30351 ChEMBL CHEMBL39879 
RTECS number DW1750000 Jmol-3D images Image 1 - c1ccnc(c1)c2ccccn2
Properties Molecular formula C10H8N2 Molar mass 156.18 g mol−1 Melting point 70-73 °C
Boiling point 273 °C
Structure Dipole moment 0 D Hazards R-phrases 25 S-phrases 36/37-45 Main hazards toxic Related compounds Related compounds 4,4'-Bipyridine
Pyridine
Phenanthroline
Terpyridine
Biphenyl (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references 2,2'-Bipyridine is a organic compound with the formula (C10H8N2). This colorless solid, commonly abbreviated bipy or bpy (pronounced "bip-ee"), is an important isomer of the bipyridine family. It is a bidentate chelating ligand, forming complexes with many transition metals. Ruthenium complex and platinum complexes of bipy exhibit intense luminescence, which may have practical applications.
Contents
Preparation and general properties
It is prepared by the dehydrogenation of pyridine using Raney nickel:[1]
Although uncoordinated bipyridine is often drawn with its nitrogen atoms in cis conformation, the lowest energy conformation both in solid state and in solution is in fact coplanar, with nitrogen atoms in trans position. Only in acidic solution bipyridine adopts a cis conformation.[2] The related N-heterocyclic ligand phenanthroline does not have the same conformational flexibility and tends to bind metal ions more strongly.
Reflecting the popularity of this ligand design, many substituted variants of bipy have been described.[3][4]
Coordination chemistry
Illustrative complexes
- Mo(CO)4(bipy), derived from Mo(CO)6.
- RuCl2(bipy)2,[5] a useful precursor to mixed ligand complexes.
- [Ru(bipy)3]Cl2, a well known luminophore.
- [Fe(bipy)3]2+ is used for the colorimetric analysis of iron ions.
Tris-bipy complexes
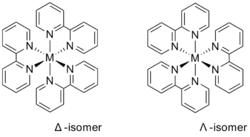
Bipyridine complexes absorb intensely in the visible part of the spectrum. The electronic transitions are attributed to metal-to-ligand charge transfer (MLCT). In the "tris(bipy) complexes" three bipyridine molecules coordinate to a metal ion, written as [M(bipy)3]n+ (M = metal ion; Cr, Fe, Co, Ru, Rh and so on; bipy = 2,2'-bipyridine). These complexes have six-coordinated, octahedral structures and two enantiomers as follows:
These and other homoleptic tris-2,2'-bipy complexes of many transition metals are electroactive. Often, both the metal centred and ligand centred electrochemical reactions are reversible one-electron reactions that can be observed by cyclic voltammetry. Under strongly reducing conditions, most tris(bipy) complexes can be reduced to neutral derivatives containing bipy- ligands. Examples include M(bipy)3, where M = Al, Cr, Si.
References
- ^ Sasse, W. H. F. (1973), "2,2’-Bipyridine", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv5p0102; Coll. Vol. 5: 102
- ^ Göller, A.; Grummt, U.-W.; "Torsional barriers in biphenyl, 2,2'-bipyridine and 2-phenylpyridine", Chem. Phys. Lett. 2000, 321, 399-405.
- ^ Smith, A. P.; Lamba, J. J. S.; Fraser, C. L. (2004), "Efficient Synthesis of Halomethyl-2,2'-Bipyridines: 4,4'-Bis(chloromethyl)-2,2'-Bipyridine", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=v78p0082; Coll. Vol. 10: 107
- ^ Smith, A. P.; Savage, S. A.; Love, J.; Fraser, C. L. (2004), "Synthesis of 4-, 5-, and 6-Methyl-2,2'-Bipyridine by a Negishi Cross-Coupling Strategy", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=v78p0051; Coll. Vol. 10: 517
- ^ Lay, P. A.; Sargeson, A. M.; Taube, H. (1986). "cis-Bis(2,2’-Bipyridine-N,N’) Complexes of Ruthenium(III)/(II) and Osmium(III)/(II)". Inorg. Synth. 24: 291–299. doi:10.1002/9780470132555.ch78.
Categories:- Chelating agents
- Bipyridines
Wikimedia Foundation. 2010.