- Methylene diphenyl diisocyanate
-
4,4'-methylene diphenyl diisocyanate 
 1-isocyanato-4-[(4-isocyanatophenyl)methyl] benzeneOther names
1-isocyanato-4-[(4-isocyanatophenyl)methyl] benzeneOther namesIdentifiers CAS number 101-68-8 
PubChem 7570 ChemSpider 7289 
ChEBI CHEBI:53218 
RTECS number NQ9350000 Jmol-3D images Image 1 - O=C=N\c1ccc(cc1)Cc2ccc(\N=C=O)cc2
Properties Molecular formula C15H10N2O2 Molar mass 250.25 g/mol Appearance white or pale yellow solid Density 1.230 g/cm3, solid Melting point 40 °C (313 K)
Boiling point 314 °C (587 K)
Solubility in water Reacts Hazards EU classification Harmful (Xn) R-phrases R20, R36/37/38, R42/43 S-phrases (S1/2), S23, S36/37, S45 Flash point 212–214 °C (Cleveland open cup) Related compounds Related Isocyanates Toluene diisocyanate
Naphthalene diisocyanate
Hexamethylene diisocyanate
Isophorone diisocyanateRelated compounds Polyurethane  diphenyl diisocyanate (verify) (what is:
diphenyl diisocyanate (verify) (what is:  /
/ ?)
?)
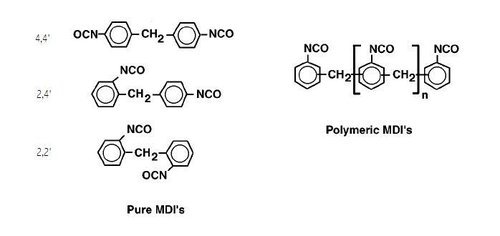
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Methylene diphenyl diisocyanate, most often abbreviated as MDI, is an aromatic diisocyanate. It exists in three isomers, 2,2'-MDI, 2,4'-MDI, and 4,4'-MDI, but the 4,4' isomer is most widely used. This isomer is also known as Pure MDI. MDI reacts with polyols in the manufacture of polyurethane. It is the most produced diisocyanate, accounting for 61.3% of the global market in the year 2000.[1]
Contents
Production
Total world production of MDI and polymeric MDI is over 2 million tonnes per year (Mt/a). Major producers include BASF, Bayer, BorsodChem, Dow, Huntsman, Mitsui, Nippon Polyurethane Industry and Yantai Wanhua. All major producers of MDI are members of the International Isocyanate Institute, whose aim is the promotion of the safe handling of MDI and TDI in the workplace, community and environment.
MDI is prepared by "phosgenation" of a diamine precursor. These diamines are treated with phosgene to form an MDI. The isomer ratio is determined by the isomeric composition of the diamine. Distillation of the MDI mixture give Polymeric MDI (a mixture of oligomeric polyisocyanates) and an MDI isomer mixture which has a low 2,4' isomer content. Further purification entails fractionation of the MDI isomer mixture.[2]
Reactivity of the isocyanate group
The positions of the isocyanate groups influences their reactivity. In 4,4'-MDI, the two isocyanate groups are equivalent but in 2,4'-MDI the two groups display highly differing reactivities. The group at the 4-position is approximately four times more reactive than the group at the 2-position.[1]
Applications
The major application of 4,4'-MDI is the production of rigid polyurethane. Typically, one tonne of polyurethane foam needs 0.616 tonne of MDI and 0.386 tonne of polyol, with 0.054 tonne pentane as a blowing agent.[citation needed] These rigid polyurethane foams are good thermal insulators and used in nearly all freezers and refrigerators worldwide, as well as buildings. Typical polyols used are polyethylene adipate (a polyester) and poly(tetramethylene ether) glycol (a polyether).
4,4'-MDI is also used as an industrial strength adhesive, which is available to end consumers as various high-strength bottled glue preparations.[3]
Safety
MDI is the least hazardous of the commonly available isocyanates but is not benign.[4] Its very low vapour pressure reduces its hazards during handling compared to the other major isocyanates (TDI, HDI). However, it, like the other isocyanates, is an allergen and sensitizer. Persons developing sensitivity to isocyanates may have dangerous systemic reactions to extremely small exposures, including respiratory failure. Handling MDI requires strict engineering controls and personal protective equipment.[5] Compared to other organic cyanates, MDI has a relatively low human toxicity. It is potentially violently reactive material toward water and other nucleophiles.
References
- ^ a b Randall, David; Lee, Steve (2002). The Polyurethanes Book. New York: Wiley. ISBN 0-470-85041-8.
- ^ Christian Six and Frank Richter "Isocyanates, Organic" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a14_611
- ^ US patent 6884904, Smith, Andrea Karen; Goddard, Richard Joseph; Paulsen, Evelyn Jennifer Lin, "MDI-based polyurethane prepolymer with low monomeric MDI content", issued 2005-04-26
- ^ Allport DC, Gilbert, DS and Outterside SM (eds) (2003). MDI and TDI: safety, health & the environment: a source book and practical guide. Chichester, Wiley. http://eu.wiley.com/WileyCDA/WileyTitle/productCd-0471958123.html
- ^ Almaguer, Daniel; et al. (September 2006). "Preventing Asthma and Death from MDI Exposure During Spray-on Truck Bed Liner and Related Applications". NIOSH Alert. The National Institute for Occupational Safety and Health. http://www.cdc.gov/niosh/docs/2006-149/. Retrieved November 10, 2008.
External links
- International Chemical Safety Card 0298
- IARC Monograph: "4,4'-Methylenediphenyl Diisocyanate"
- NIOSH Safety and Health Topic: Isocyanates, from the website of the National Institute for Occupational Safety and Health (NIOSH)
- Hazards of TDI, MDI, and HDI
- Isofact American Chemistry Council Diisocyanates Panel
- Azom Chemical database on Polyurethane chemistry
- MDI and the Environment - 2005 presentation by Center for the Polyurethanes Industry
- International Isocyanate Institute
- Concise International Chemical Assessment Document 27
Categories:- Isocyanates
- Monomers
- IARC Group 3 carcinogens
Wikimedia Foundation. 2010.