- Titanium tetrachloride
-
Titanium tetrachloride  Titanium tetrachlorideSystematic nameTetrachlorotitaniumOther namesTitanium(IV) chloride
Titanium tetrachlorideSystematic nameTetrachlorotitaniumOther namesTitanium(IV) chlorideIdentifiers CAS number 7550-45-0 
PubChem 24193 ChemSpider 22615 
EC number 231-441-9 UN number 1838 MeSH Titanium+tetrachloride RTECS number XR1925000 Jmol-3D images Image 1 - Cl[Ti](Cl)(Cl)Cl
Properties Molecular formula TiCl4 Molar mass 189.679 g mol-1 Exact mass 187.823357881 g mol-1 Appearance Colourless liquid Density 1.726 g cm-3 Melting point -25 °C, 248.6 K, -12 °F
Boiling point 135 °C, 408 K, 275 °F
Solubility in water Reacts Vapor pressure 1.3 kPa (at 20 °C) Viscosity 827 μPa s Structure Coordination
geometryTetragonal Molecular shape Tetrahedral Dipole moment 0 D Thermochemistry Std enthalpy of
formation ΔfHo298-804.16 kJ mol Standard molar
entropy So298221.93 J K–1 mol–1 Hazards MSDS MSDS EU Index 022-001-00-5 EU classification  C
CR-phrases R14, R34 S-phrases (S1/2), S7/8, S26, S36/37/39, S45 NFPA 704 Related compounds Related titanium chlorides Titanium(II) chloride
Related compounds Hafnium(IV) chloride
Titanium(IV) bromide
Titanium(IV) fluoride
Titanium(IV) iodide
Zirconium(IV) chloride tetrachloride (verify) (what is:
tetrachloride (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Titanium tetrachloride is the inorganic compound with the formula TiCl4. It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. TiCl4 is an unusual example of a metal halide that is highly volatile. Upon contact with humid air, it forms spectacular opaque clouds of titanium dioxide (TiO2) and hydrogen chloride (HCl).
Contents
Properties and structure
TiCl4 is a dense, colourless distillable liquid, although crude samples may be yellow or even red-brown. It is one of the rare transition metal halides that is a liquid at room temperature, VCl4 being another example. This property reflects the fact that TiCl4 is molecular; that is, each TiCl4 molecule is relatively weakly associated with its neighbours. Most metal chlorides are polymers, wherein the chloride atoms bridge between the metals. The attraction between the individual TiCl4 molecules is weak, primarily van der Waals forces, and these weak interactions result in low melting and boiling points, similar to those of CCl4.
Ti4+ has a "closed" electronic shell, with the same number of electrons as the inert gas argon. The tetrahedral structure for TiCl4 is consistent with its description as a d0 metal center (Ti4+) surrounded by four identical ligands. This configuration leads to highly symmetrical structures, hence the tetrahedral shape of the molecule. TiCl4 adopts similar structures to TiBr4 and TiI4; the three compounds share many similarities. TiCl4 and TiBr4 react to give mixed halides TiCl4-xBrx, where x = 0, 1, 2, 3, 4. Magnetic resonance measurements also indicate that halide exchange is also rapid between TiCl4 and VCl4.[1]
TiCl4 is soluble in toluene and chlorocarbons, as are other non-polar species. Evidence exists that certain arenes form complexes of the type [(C6R6)TiCl3]+. TiCl4 reacts exothermically with donor solvents such as THF to give hexacoordinated adducts.[2] Bulkier ligands (L) give pentacoordinated adducts TiCl4L.
Production
TiCl4 is produced by the chloride process, which involves the reduction of titanium oxide ores, typically ilmenite (FeTiO3) with carbon under flowing chlorine at 900 °C. Impurities are removed by distillation.
- 2 FeTiO3 + 7 Cl2 + 6 C → 2 TiCl4 + 2 FeCl3 + 6 CO
The coproduction of FeCl3 is undesirable, which has motivated the development of alternative technologies. Instead of directly using ilmenite, "rutile slag" is used. This material, an impure form of TiO2, is derived from ilmenite by removal of iron, either using carbon reduction or extraction with sulfuric acid. Crude TiCl4 contains a variety of other volatile halides, including vanadyl chloride (VOCl3), silicon tetrachloride (SiCl4), and tin tetrachloride (SnCl4), which must be separated.
Applications
Production of titanium metal
The world's supply of titanium metal, about 4M tons per year, is made from TiCl4. The conversion takes place by the reduction of the chloride with magnesium metal, and yields titanium metal and magnesium chloride. This procedure is the final step of the Kroll process:
- 2 Mg + TiCl4 → 2 MgCl2 + Ti
Liquid sodium has also been used instead of magnesium as the reducing agent.
Production of titanium dioxide
Around 90% of the TiCl4 production is used to make the pigment titanium dioxide (TiO2). The conversion involves hydrolysis of TiCl4, a process that forms hydrogen chloride:[3]
- TiCl4 + 2 H2O → TiO2 + 4 HCl
In some cases, TiCl4 is oxidised directly with oxygen:
- TiCl4 + O2 → TiO2 + 2 Cl2
Chemical reactions
Titanium tetrachloride is a versatile reagent that forms diverse derivatives including those illustrated below.
The most noteworthy reaction of TiCl4 is its easy hydrolysis, signaled by the release of corrosive hydrogen chloride and the formation of titanium oxides and oxychlorides, as described above for the production of TiO2. In the past titanium tetrachloride has also been used to create naval smokescreens. The hydrogen chloride immediately absorbs more water to form tiny droplets of hydrochloric acid, which (depending on humidity) may absorb still more water, to produce large droplets that efficiently scatter light. In addition, the highly refractive titanium dioxide is also an efficient light scatterer. Because of the corrosiveness of its smoke, however, TiCl4 is no longer used.
Alcohols react with TiCl4 to give the corresponding alkoxides with the formula [Ti(OR)4]n (R = alkyl, n = 1, 2, 4). As indicated by their formula, these alkoxides can adopt complex structures ranging from monomers to tetramers. Such compounds are useful in materials science as well as organic synthesis. A well known derivative is titanium isopropoxide, which is a monomer.
Organic amines react with TiCl4 to give complexes containing amido (R2N--containing) and imido (RN2--containing) complexes. With ammonia, titanium nitride is formed. An illustrative reaction is the synthesis of tetrakis(dimethylamido)titanium Ti(NMe2)4, a yellow, benzene-soluble liquid:[4] This molecule is tetrahedral, with planar nitrogen centers.[5]
- 4 LiNMe2 + TiCl4 → 4 LiCl + Ti(NMe2)4
Complexes with simple ligands
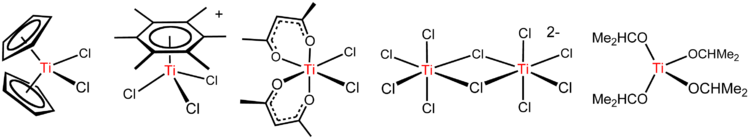
TiCl4 is a Lewis acid as implicated by its tendency to hydrolyze. With the ether THF, TiCl4 reacts to give yellow crystals of TiCl4(THF)2. With chloride salts, TiCl4 reacts to form sequentially [Ti2Cl9]−, [Ti2Cl10]2− (see figure above), and [TiCl6]2−.[6] Interestingly, the reaction of chloride ions with TiCl4 depends on the counterion. NBu4Cl and TiCl4 gives the pentacoordinate complex NBu4TiCl5, whereas smaller NEt4+ gives (NEt4)2Ti2Cl10. These reactions highlight the influence of electrostatic forces on the structures of compounds with highly ionic bonding.
Redox
Reduction of TiCl4 with aluminium results in one-electron reduction. The trichloride (TiCl3) and tetrachloride have contrasting properties: the trichloride is a solid, being a coordination polymer, and is paramagnetic. When the reduction is conducted in THF solution, the Ti(III) product converts to the light-blue adduct TiCl3(thf)3.
Organometallic chemistry
Main article: Organotitanium compoundThe organometallic chemistry of titanium typically starts from TiCl4. An important reaction involves sodium cyclopentadienyl to give titanocene dichloride, TiCl2(C5H5)2. This compound and many of its derivatives are precursors to Ziegler-Natta catalysts. Tebbe's reagent, useful in organic chemistry, is an aluminium-containing derivative of titanocene that arises from the reaction of titanocene dichloride with trimethylaluminium. It is used for the "olefination" reactions.
Arenes, such as C6(CH3)6 react to give the piano-stool complexes [Ti(C6R6)Cl3]+ (R = H, CH3; see figure above).[7] This reaction illustrates the high Lewis acidity of the TiCl3+ entity, which is generated by abstraction of chloride from TiCl4 by AlCl3.
Reagent in organic synthesis
TiCl4 finds limited but diverse use in organic synthesis, capitalizing on its Lewis acidity and its oxophilicity.[8] Illustrative is the Mukaiyama aldol reaction. Key to this application is the tendency of TiCl4 to activate aldehydes (RCHO) by formation of adducts such (RCHO)TiCl4OC(H)R. It is also used in the McMurry reaction in conjunction with zinc, LiAlH4. These reducing agents generate Ti(III) derivatives that couple ketones, leading to alkenes.
Toxicity and safety considerations
Hazards posed by titanium tetrachloride generally arise from the release of hydrogen chloride (HCl). TiCl4 is a strong Lewis acid, exothermically forming adducts with even weak bases such as THF and explosively with water, releasing HCl.
References
- ^ S. P. Webb, M. S. Gordon (1999). "Intermolecular Self-Interactions of the Titanium Tetrahalides TiX4 (X = F, Cl, Br)". J. Am. Chem. Soc. 121 (11): 2552–2560. doi:10.1021/ja983339i.
- ^ L. E. Manzer; Deaton, Joe; Sharp, Paul; Schrock, R. R. (1982). "Tetrahydrofuran Complexes of Selected Early Transition Metals". Inorganic Synthesis. Inorganic Syntheses 21: 135–40. doi:10.1002/9780470132524.ch31. ISBN 9780470132524.
- ^ Hans G. Völz et al. “Pigments, Inorganic” in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2006. doi:10.1002/14356007.a20_243.pub2
- ^ D. C. Bradey, M. Thomas (1960). "Some Dialkylamino-derivatives of Titanium and Zirconium". Journal of the Chemical Society: 3857–3861. doi:10.1039/JR9600003857.
- ^ M. E. Davie, T. Foerster, S. Parsons, C. Pulham, D. W. H. Rankin, B. A. Smart (2006). "The Crystal Structure of Tetrakis(dimethylamino)titanium(IV)". Polyhedron 25 (4): 923–929. doi:10.1016/j.poly.2005.10.019.
- ^ C. S. Creaser , J. A. Creighton (1975). "Pentachloro- and Pentabromotitanate(IV) ions". Journal of the Chemical Society, Dalton Transactions (14): 1402–1405. doi:10.1039/DT9750001402.
- ^ F. Calderazzo, I. Ferri, G. Pampaloni, S. Troyanov (1996). "η6-Arene Derivatives of Titanium(IV), Zirconium(IV) and Hafnium(IV)". Journal of Organometallic Chemistry 518: 189–196. doi:10.1016/0022-328X(96)06194-3.
- ^ L.-L. Gundersen, F. Rise, K. Undheim (2004). "Titanium(IV) chloride". Encyclopedia of Reagents for Organic Synthesis (L. Paquette ed.). New York: J. Wiley & Sons.
General reading
- Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Oxford: Butterworth-Heinemann. ISBN 0080379419.
External links
- Titanium tetrachloride: Health Hazard Information
- ESIS: European chemical Substances Information System
- NIST Standard Reference Database
- ChemSub Online: Titanium tetrachloride
Titanium compounds Categories:- Titanium compounds
- Chlorides
- Metal halides
- Reagents for organic chemistry
Wikimedia Foundation. 2010.