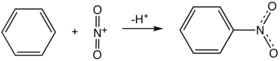- Nitrobenzene
-
Nitrobenzene 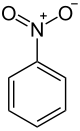
 Other namesNitrobenzol
Other namesNitrobenzol
Oil of mirbaneIdentifiers CAS number 98-95-3 
PubChem 7416 ChemSpider 7138 
UNII E57JCN6SSY 
KEGG C06813 
ChEBI CHEBI:27798 
ChEMBL CHEMBL15750 
RTECS number QJ0525000 Jmol-3D images Image 1 - c1ccc(cc1)[N+](=O)[O-]
Properties Molecular formula C6H5NO2 Molar mass 123.06 g/mol Appearance yellowish liquid Density 1.199 g/cm3 Melting point 5.7 °C
Boiling point 210.9 °C
Solubility in water 0.19 g/100 ml at 20 °C Hazards EU classification Toxic (T)
Carc. Cat. 3
Repr. Cat. 3
Dangerous for the environment (N)
Flammable(F)R-phrases R10, R23/24/25, R40,
R48/23/24, R51/53, R62S-phrases (S1/2), S28, S36/37,
S45, S61NFPA 704 Flash point 88 °C Autoignition
temperature525 °C Related compounds Related compounds Aniline
Benzenediazonium chloride
Nitrosobenzene (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Nitrobenzene is an organic compound with the chemical formula C6H5NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale as a precursor to aniline. Although occasionally used as a flavoring or perfume additive, nitrobenzene is highly toxic in large quantities. In the laboratory, it is occasionally used as a solvent, especially for electrophilic reagents.
Contents
Production
Nitrobenzene is prepared by nitration of benzene with a mixture of concentrated sulfuric acid, water, and nitric acid. This mixture is sometimes called "mixed acid." The production of nitrobenzene is one of the more dangerous processes conducted in the chemical industry because of the exothermicity of the reaction (ΔH = −117 kJ/mol).[1]
World capacity for nitrobenzene in 1985 was about 1.7×106 tonnes.[1]
Mechanism of nitration
Main article: nitrationThe reaction pathway entails formation of an adduct between the Lewis acidic nitronium ion (NO2+) and benzene. The nitronium ion is generated in situ by the reaction of nitric acid and an acidic dehydration agent, typically sulfuric acid:
Uses
Approximately 95% of nitrobenzene is consumed in the production of aniline,[1] which is a precursor to rubber chemicals, pesticides, dyes, explosives, and pharmaceuticals.
Specialized applications
Nitrobenzene is also used in shoe and floor polishes, leather dressings, paint solvents, and other materials to mask unpleasant odors. Redistilled, as oil of mirbane, nitrobenzene has been used as an inexpensive perfume for soaps. A significant merchant market for nitrobenzene is its use in the production of the analgesic paracetamol (also known as acetaminophen) (Mannsville 1991).[2] Nitrobenzene is also used in Kerr cells, as it has an unusually large Kerr constant.
Organic reactions
Aside from its conversion to aniline, nitrobenzene is readily converted to related derivatives such as azobenzene,[3] nitrosobenzene,[4] and phenylhydroxylamine.[5] The nitro- group is deactivating, thus substitution tends to occur at the meta-position.
Safety
Nitrobenzene is highly toxic (Threshold Limit Value 5 mg/m3) and readily absorbed through the skin.
Prolonged exposure may cause serious damage to the central nervous system, impair vision, cause liver or kidney damage, anemia and lung irritation. Inhalation of fumes may induce headache, nausea, fatigue, dizziness, cyanosis, weakness in the arms and legs, and in rare cases may be fatal. The oil is readily absorbed through the skin and may increase heart rate, cause convulsions or rarely death. Ingestion may similarly cause headaches, dizziness, nausea, vomiting and gastrointestinal irritation, loss of limbs and also causes internal bleeding.
Nitrobenzene is considered a likely human carcinogen by the United States Environmental Protection Agency,[6] and is classified by the IARC as a Group 2B carcinogen which is "possibly carcinogenic to humans".[7] It has been shown to cause liver, kidney, and thyroid adenomas and carcinomas in rats.
References
- ^ a b c Gerald Booth (2007). "Nitro Compounds, Aromatic". In: Ullmann's Encyclopedia of Industrial Chemistry. John Wiley & Sons: New York. doi:10.1002/14356007.a17_411
- ^ Bhattacharya A.; Purohit V. C.; Suarez, V.; Tichkule, R; Parmer, G.; Rinaldi, F. (2006). "One-step reductive amidation of nitro arenes: application in the synthesis of Acetaminophen". Tetrahedron Letters 47 (11): 1861–1864. doi:10.1016/j.tetlet.2005.09.196. http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6THS-4J555V1-1&_user=10&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=fe6796487e91354be14cc99413b05808.
- ^ Bigelow, H. E.; Robinson, D. B. (1955), "Azobenzene", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV3P0103; Coll. Vol. 3: 103
- ^ G. H. Coleman, C. M. McCloskey, F. A. Stuart, "Nitrosobenzene", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV3P0668; Coll. Vol. 3: 668
- ^ O. Kamm, "β-Phenylhydroxylamine", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv1p0445; Coll. Vol. 1: 445
- ^ http://cfpub.epa.gov/ncea/iris/index.cfm?fuseaction=iris.showQuickView&substance_nmbr=0079
- ^ Agents Classified by the IARC Monographs, International Agency for Research on Cancer
External links
Categories:- Nitrobenzenes
- Nitro solvents
- IARC Group 2B carcinogens
- Nonlinear optical materials
Wikimedia Foundation. 2010.