- Ozone depletion
-
Ozone depletion describes two distinct but related phenomena observed since the late 1970s: a steady decline of about 4% per decade in the total volume of ozone in Earth's stratosphere (the ozone layer), and a much larger springtime decrease in stratospheric ozone over Earth's polar regions. The latter phenomenon is referred to as the ozone hole. In addition to these well-known stratospheric phenomena, there are also springtime polar tropospheric ozone depletion events.
The details of polar ozone hole formation differ from that of mid-latitude thinning, but the most important process in both is catalytic destruction of ozone by atomic halogens.[1] The main source of these halogen atoms in the stratosphere is photodissociation of man-made halocarbon refrigerants (CFCs, freons, halons). These compounds are transported into the stratosphere after being emitted at the surface. [2] Both types of ozone depletion were observed to increase as emissions of halo-carbons increased.
CFCs and other contributory substances are referred to as ozone-depleting substances (ODS). Since the ozone layer prevents most harmful UVB wavelengths (280–315 nm) of ultraviolet light (UV light) from passing through the Earth's atmosphere, observed and projected decreases in ozone have generated worldwide concern leading to adoption of the Montreal Protocol that bans the production of CFCs, halons, and other ozone-depleting chemicals such as carbon tetrachloride and trichloroethane. It is suspected that a variety of biological consequences such as increases in skin cancer, cataracts,[3] damage to plants, and reduction of plankton populations in the ocean's photic zone may result from the increased UV exposure due to ozone depletion.
Ozone cycle overview
Three forms (or allotropes) of oxygen are involved in the ozone-oxygen cycle: oxygen atoms (O or atomic oxygen), oxygen gas (O2 or diatomic oxygen), and ozone gas (O3 or triatomic oxygen). Ozone is formed in the stratosphere when oxygen molecules photodissociate after absorbing an ultraviolet photon whose wavelength is shorter than 240 nm. This converts a single O2 into two atomic oxygen ions. The atomic oxygen ions then combine with separate O2 molecules to create two O3 molecules. These ozone molecules absorb UV light between 310 and 200 nm, following which ozone splits into a molecule of O2 and an oxygen atom. The oxygen atom then joins up with an oxygen molecule to regenerate ozone. This is a continuing process which terminates when an oxygen atom "recombines" with an ozone molecule to make two O2 molecules.
O + O3 → 2 O2 chemical equation
The overall amount of ozone in the stratosphere is determined by a balance between photochemical production and recombination.
Ozone can be destroyed by a number of free radical catalysts, the most important of which are the hydroxyl radical (OH·), the nitric oxide radical (NO·), the atomic chlorine ion (Cl·) and the atomic bromine ion (Br·). All of these have both natural and man-made sources; at the present time, most of the OH· and NO· in the stratosphere is of natural origin, but human activity has dramatically increased the levels of chlorine and bromine. These elements are found in certain stable organic compounds, especially chlorofluorocarbons (CFCs), which may find their way to the stratosphere without being destroyed in the troposphere due to their low reactivity. Once in the stratosphere, the Cl and Br atoms are liberated from the parent compounds by the action of ultraviolet light, e.g.
CFCl3 + electromagnetic radiation → CFCl2 + Cl
The Cl and Br atoms can then destroy ozone molecules through a variety of catalytic cycles. In the simplest example of such a cycle,[4] a chlorine atom reacts with an ozone molecule, taking an oxygen atom with it (forming ClO) and leaving a normal oxygen molecule. The chlorine monoxide (i.e., the ClO) can react with a second molecule of ozone (i.e., O3) to yield another chlorine atom and two molecules of oxygen. The chemical shorthand for these gas-phase reactions is:
- Cl + O3 → ClO + O2 – The chlorine atom changes an ozone molecule to ordinary oxygen
- ClO + O3 → Cl + 2 O2 – The ClO from the previous reaction destroys a second ozone molecule and recreates the original chlorine atom, which can repeat the first reaction and continue to destroy ozone
The overall effect is a decrease in the amount of ozone. More complicated mechanisms have been discovered that lead to ozone destruction in the lower stratosphere as well.
A single chlorine atom would keep on destroying ozone (thus a catalyst) for up to two years (the time scale for transport back down to the troposphere) were it not for reactions that remove them from this cycle by forming reservoir species such as hydrogen chloride (HCl) and chlorine nitrate (ClONO2). On a per atom basis, bromine is even more efficient than chlorine at destroying ozone, but there is much less bromine in the atmosphere at present. As a result, both chlorine and bromine contribute significantly to the overall ozone depletion. Laboratory studies have shown that fluorine and iodine atoms participate in analogous catalytic cycles. However, in the Earth's stratosphere, fluorine atoms react rapidly with water and methane to form strongly bound HF, while organic molecules which contain iodine react so rapidly in the lower atmosphere that they do not reach the stratosphere in significant quantities. Furthermore, a single chlorine atom is able to react with 100,000 ozone molecules. This fact plus the amount of chlorine released into the atmosphere by chlorofluorocarbons (CFCs) yearly demonstrates how dangerous CFCs are to the environment.[5]
Observations on ozone layer depletion
The most pronounced decrease in ozone has been in the lower stratosphere. However, the ozone hole is most usually measured not in terms of ozone concentrations at these levels (which are typically of a few parts per million) but by reduction in the total column ozone, above a point on the Earth's surface, which is normally expressed in Dobson units, abbreviated as "DU". Marked decreases in column ozone in the Antarctic spring and early summer compared to the early 1970s and before have been observed using instruments such as the Total Ozone Mapping Spectrometer (TOMS).[6]
 Lowest value of ozone measured by TOMS each year in the ozone hole.
Lowest value of ozone measured by TOMS each year in the ozone hole.
Reductions of up to 70% in the ozone column observed in the austral (southern hemispheric) spring over Antarctica and first reported in 1985 (Farman et al. 1985) are continuing.[7] Through the 1990s, total column ozone in September and October have continued to be 40–50% lower than pre-ozone-hole values. In the Arctic the amount lost is more variable year-to-year than in the Antarctic. The greatest declines, up to 30%, are in the winter and spring, when the stratosphere is colder.
Reactions that take place on polar stratospheric clouds (PSCs) play an important role in enhancing ozone depletion.[8] PSCs form more readily in the extreme cold of Antarctic stratosphere. This is why ozone holes first formed, and are deeper, over Antarctica. Early models failed to take PSCs into account and predicted a gradual global depletion, which is why the sudden Antarctic ozone hole was such a surprise to many scientists.[citation needed]
In middle latitudes it is preferable to speak of ozone depletion rather than holes. Declines are about 3% below pre-1980 values for 35–60°N and about 6% for 35–60°S. In the tropics, there are no significant trends.[9]
Ozone depletion also explains much of the observed reduction in stratospheric and upper tropospheric temperatures.[10][11] The source of the warmth of the stratosphere is the absorption of UV radiation by ozone, hence reduced ozone leads to cooling. Some stratospheric cooling is also predicted from increases in greenhouse gases such as CO2; however the ozone-induced cooling appears to be dominant.[citation needed]
Predictions of ozone levels remain difficult. The World Meteorological Organization Global Ozone Research and Monitoring Project—Report No. 44 comes out strongly in favor for the Montreal Protocol, but notes that a UNEP 1994 Assessment overestimated ozone loss for the 1994–1997 period.
Chemicals in the atmosphere
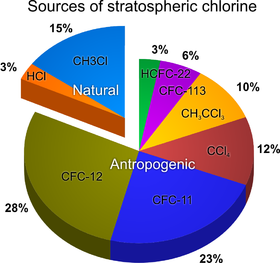
Chlorofluorocarbons (CFCs) and other halogenated ozone depleting substances (ODS) are mainly responsible for man-made chemical ozone depletion. The total amount of effective halogens (chlorine and bromine) in the stratosphere can be calculated and are known as the equivalent effective stratospheric chlorine (EESC).[12]
CFCs were invented by Thomas Midgley, Jr. in the 1920s. They were used in air conditioning/cooling units, as aerosol spray propellants prior to the 1980s, and in the cleaning processes of delicate electronic equipment. They also occur as by-products of some chemical processes. No significant natural sources have ever been identified for these compounds — their presence in the atmosphere is due almost entirely to human manufacture. As mentioned in the ozone cycle overview above, when such ozone-depleting chemicals reach the stratosphere, they are dissociated by ultraviolet light to release chlorine atoms. The chlorine atoms act as a catalyst, and each can break down tens of thousands of ozone molecules before being removed from the stratosphere. Given the longevity of CFC molecules, recovery times are measured in decades. It is calculated that a CFC molecule takes an average of about five to seven years to go from the ground level up to the upper atmosphere, and it can stay there for about a century, destroying up to one hundred thousand ozone molecules during that time.[13][verification needed]
Verification of observations
Scientists have been increasingly able to attribute the observed ozone depletion to the increase of man-made (anthropogenic) halogen compounds from CFCs by the use of complex chemistry transport models and their validation against observational data (e.g. SLIMCAT, CLaMS — Chemical Lagrangian Model of the Stratosphere). These models work by combining satellite measurements of chemical concentrations and meteorological fields with chemical reaction rate constants obtained in lab experiments. They are able to identify not only the key chemical reactions but also the transport processes which bring CFC photolysis products into contact with ozone.
The ozone hole and its causes
 Ozone hole in North America during 1984 (abnormally warm reducing ozone depletion) and 1997 (abnormally cold resulting in increased seasonal depletion). Source: NASA[14]
Ozone hole in North America during 1984 (abnormally warm reducing ozone depletion) and 1997 (abnormally cold resulting in increased seasonal depletion). Source: NASA[14]
The Antarctic ozone hole is an area of the Antarctic stratosphere in which the recent ozone levels have dropped to as low as 33% of their pre-1975 values. The ozone hole occurs during the Antarctic spring, from September to early December, as strong westerly winds start to circulate around the continent and create an atmospheric container. Within this polar vortex, over 50% of the lower stratospheric ozone is destroyed during the Antarctic spring.[15]
As explained above, the primary cause of ozone depletion is the presence of chlorine-containing source gases (primarily CFCs and related halocarbons). In the presence of UV light, these gases dissociate, releasing chlorine atoms, which then go on to catalyze ozone destruction. The Cl-catalyzed ozone depletion can take place in the gas phase, but it is dramatically enhanced in the presence of polar stratospheric clouds (PSCs).[16]
These polar stratospheric clouds(PSC) form during winter, in the extreme cold. Polar winters are dark, consisting of 3 months without solar radiation (sunlight). The lack of sunlight contributes to a decrease in temperature and the polar vortex traps and chills air. Temperatures hover around or below −80 °C. These low temperatures form cloud particles. There are three types of PSC clouds; nitric acid trihydrate clouds, slowly cooling water-ice clouds, and rapid cooling water-ice(nacerous) clouds; that provide surfaces for chemical reactions that lead to ozone destruction.[17]
The photochemical processes involved are complex but well understood. The key observation is that, ordinarily, most of the chlorine in the stratosphere resides in stable "reservoir" compounds, primarily hydrochloric acid (HCl) and chlorine nitrate (ClONO2). During the Antarctic winter and spring, however, reactions on the surface of the polar stratospheric cloud particles convert these "reservoir" compounds into reactive free radicals (Cl and ClO). The clouds can also remove NO2 from the atmosphere by converting it to nitric acid, which prevents the newly formed ClO from being converted back into ClONO2.
The role of sunlight in ozone depletion is the reason why the Antarctic ozone depletion is greatest during spring. During winter, even though PSCs are at their most abundant, there is no light over the pole to drive the chemical reactions. During the spring, however, the sun comes out, providing energy to drive photochemical reactions, and melt the polar stratospheric clouds, releasing the trapped compounds. Warming temperatures near the end of spring break up the vortex around mid-December. As warm, ozone-rich air flows in from lower latitudes, the PSCs are destroyed, the ozone depletion process shuts down, and the ozone hole closes.[18]
Most of the ozone that is destroyed is in the lower stratosphere, in contrast to the much smaller ozone depletion through homogeneous gas phase reactions, which occurs primarily in the upper stratosphere.[19]
Interest in ozone layer depletion
While the effect of the Antarctic ozone hole in decreasing the global ozone is relatively small, estimated at about 4% per decade, the hole has generated a great deal of interest because:
- The decrease in the ozone layer was predicted in the early 1980s to be roughly 7% over a 60 year period.[citation needed]
- The sudden recognition in 1985 that there was a substantial "hole" was widely reported in the press. The especially rapid ozone depletion in Antarctica had previously been dismissed as a measurement error.[20]
- Many[who?] were worried that ozone holes might start to appear over other areas of the globe but to date the only other large-scale depletion is a smaller ozone "dimple" observed during the Arctic spring over the North Pole. Ozone at middle latitudes has declined, but by a much smaller extent (about 4–5% decrease).
- If the conditions became more severe (cooler stratospheric temperatures, more stratospheric clouds, more active chlorine), then global ozone may decrease at a much greater pace. Standard global warming theory predicts that the stratosphere will cool.[21]
- When the Antarctic ozone hole breaks up, the ozone-depleted air drifts out into nearby areas. Decreases in the ozone level of up to 10% have been reported in New Zealand in the month following the break-up of the Antarctic ozone hole.[22]
Consequences of ozone layer depletion
Since the ozone layer absorbs UVB ultraviolet light from the Sun, ozone layer depletion is expected to increase surface UVB levels, which could lead to damage, including increase in skin cancer. This was the reason for the Montreal Protocol. Although decreases in stratospheric ozone are well-tied to CFCs and there are good theoretical reasons to believe that decreases in ozone will lead to increases in surface UVB, there is no direct observational evidence linking ozone depletion to higher incidence of skin cancer in human beings. This is partly because UVA, which has also been implicated in some forms of skin cancer, is not absorbed by ozone, and it is nearly impossible to control statistics for lifestyle changes in the populace.
Increased UV
Ozone, while a minority constituent in the Earth's atmosphere, is responsible for most of the absorption of UVB radiation. The amount of UVB radiation that penetrates through the ozone layer decreases exponentially with the slant-path thickness/density of the layer. Correspondingly, a decrease in atmospheric ozone is expected to give rise to significantly increased levels of UVB near the surface.
Increases in surface UVB due to the ozone hole can be partially inferred by radiative transfer model calculations, but cannot be calculated from direct measurements because of the lack of reliable historical (pre-ozone-hole) surface UV data, although more recent surface UV observation measurement programmes exist (e.g. at Lauder, New Zealand).[23]
Because it is this same UV radiation that creates ozone in the ozone layer from O2 (regular oxygen) in the first place, a reduction in stratospheric ozone would actually tend to increase photochemical production of ozone at lower levels (in the troposphere), although the overall observed trends in total column ozone still show a decrease, largely because ozone produced lower down has a naturally shorter photochemical lifetime, so it is destroyed before the concentrations could reach a level which would compensate for the ozone reduction higher up.[citation needed]
Biological effects
The main public concern regarding the ozone hole has been the effects of increased surface UV radiation on human health. So far, ozone depletion in most locations has been typically a few percent and, as noted above, no direct evidence of health damage is available in most latitudes. Were the high levels of depletion seen in the ozone hole ever to be common across the globe, the effects could be substantially more dramatic. As the ozone hole over Antarctica has in some instances grown so large as to reach southern parts of Australia, New Zealand, Chile, Argentina, and South Africa, environmentalists have been concerned that the increase in surface UV could be significant.[24]
Ozone depletion will change all of the effects of UVB on human health, both positive and negative.
UVB (the higher energy UV radiation absorbed by ozone) is generally accepted to be a contributory factor to skin cancer and to produce Vitamin D. In addition, increased surface UV leads to increased tropospheric ozone, which is a health risk to humans.[25]
1. Basal and squamous cell carcinomas — The most common forms of skin cancer in humans, basal and squamous cell carcinomas, have been strongly linked to UVB exposure. The mechanism by which UVB induces these cancers is well understood—absorption of UVB radiation causes the pyrimidine bases in the DNA molecule to form dimers, resulting in transcription errors when the DNA replicates. These cancers are relatively mild and rarely fatal, although the treatment of squamous cell carcinoma sometimes requires extensive reconstructive surgery. By combining epidemiological data with results of animal studies, scientists have estimated that a one percent decrease in stratospheric ozone would increase the incidence of these cancers by 2%.[26]
2. Malignant melanoma — Another form of skin cancer, malignant melanoma, is much less common but far more dangerous, being lethal in about 15–20% of the cases diagnosed. The relationship between malignant melanoma and ultraviolet exposure is not yet well understood, but it appears that both UVB and UVA are involved. Experiments on fish suggest that 90 to 95% of malignant melanomas may be due to UVA and visible radiation[27] whereas experiments on opossums suggest a larger role for UVB.[26] Because of this uncertainty, it is difficult to estimate the impact of ozone depletion on melanoma incidence. One study showed that a 10% increase in UVB radiation was associated with a 19% increase in melanomas for men and 16% for women.[28] A study of people in Punta Arenas, at the southern tip of Chile, showed a 56% increase in melanoma and a 46% increase in nonmelanoma skin cancer over a period of seven years, along with decreased ozone and increased UVB levels.[29]
3. Cortical cataracts — Studies are suggestive of an association between ocular cortical cataracts and UV-B exposure, using crude approximations of exposure and various cataract assessment techniques. A detailed assessment of ocular exposure to UV-B was carried out in a study on Chesapeake Bay Watermen, where increases in average annual ocular exposure were associated with increasing risk of cortical opacity.[30] In this highly exposed group of predominantly white males, the evidence linking cortical opacities to sunlight exposure was the strongest to date. However, subsequent data from a population-based study in Beaver Dam, WI suggested the risk may be confined to men. In the Beaver Dam study, the exposures among women were lower than exposures among men, and no association was seen.[31] Moreover, there were no data linking sunlight exposure to risk of cataract in African Americans, although other eye diseases have different prevalences among the different racial groups, and cortical opacity appears to be higher in African Americans compared with whites.[32][33]
4. Increased tropospheric ozone — Increased surface UV leads to increased tropospheric ozone. Ground-level ozone is generally recognized to be a health risk, as ozone is toxic due to its strong oxidant properties. At this time, ozone at ground level is produced mainly by the action of UV radiation on combustion gases from vehicle exhausts.[citation needed]
5. Increased production of Vitamin D
Main article: Vitamin D
Vitamin D is produced in the skin by ultraviolet light. Thus, higher UV-B exposure raises human vitamin D in those deficient in it. Recent research (primarily since the Montreal protocol), shows that many humans have less than optimal vitamin D levels. In particular, the lowest quartile of vitamin D (<17.8 ng/ml), in the US population were found using information from the National Health and Nutrition Examination Survey to be associated with an increase in all cause mortality in the general population.[34] While higher level of Vitamin D are associated with higher mortality, the body has mechanisms that prevent sunlight from producing too much Vitamin D.[35]
Effects on non-human animals
A November 2010 report by scientists at the Institute of Zoology in London found that whales off the coast of California have shown a sharp rise in sun damage, and these scientists "fear that the thinning ozone layer is to blame"[36]
The study photographed and took skin biopsies from over 150 whales in the Gulf of California and found "widespread evidence of epidermal damage commonly associated with acute and severe sunburn," having cells which form when the DNA is damaged by UV radiation. The findings suggest "rising UV levels as a result of ozone depletion are to blame for the observed skin damage, in the same way that human skin cancer rates have been on the increase in recent decades."[37]
Effects on crops
An increase of UV radiation would be expected to affect crops. A number of economically important species of plants, such as rice, depend on cyanobacteria residing on their roots for the retention of nitrogen. Cyanobacteria are sensitive to UV light and would be affected by its increase.[38]
Public policy
The full extent of the damage that CFCs have caused to the ozone layer is not known and will not be known for decades; however, marked decreases in column ozone have already been observed (as explained before).
After a 1976 report by the United States National Academy of Sciences concluded that credible scientific evidence supported the ozone depletion hypothesis[39] a few countries, including the United States, Canada, Sweden, Denmark, and Norway, moved to eliminate the use of CFCs in aerosol spray cans.[40] At the time this was widely regarded as a first step towards a more comprehensive regulation policy, but progress in this direction slowed in subsequent years, due to a combination of political factors (continued resistance from the halocarbon industry and a general change in attitude towards environmental regulation during the first two years of the Reagan administration) and scientific developments (subsequent National Academy assessments which indicated that the first estimates of the magnitude of ozone depletion had been overly large). A critical DuPont manufacturing patent for Freon was set to expire in 1979. The United States banned the use of CFCs in aerosol cans in 1978.[40] The European Community rejected proposals to ban CFCs in aerosol sprays, and in the U.S., CFCs continued to be used as refrigerants and for cleaning circuit boards. Worldwide CFC production fell sharply after the U.S. aerosol ban, but by 1986 had returned nearly to its 1976 level.[40] In 1993, DuPont shut down its CFC facility.[41]
The U.S. Government's attitude began to change again in 1983, when William Ruckelshaus replaced Anne M. Burford as Administrator of the United States Environmental Protection Agency. Under Ruckelshaus and his successor, Lee Thomas, the EPA pushed for an international approach to halocarbon regulations. In 1985 20 nations, including most of the major CFC producers, signed the Vienna Convention for the Protection of the Ozone Layer which established a framework for negotiating international regulations on ozone-depleting substances. That same year, the discovery of the Antarctic ozone hole was announced, causing a revival in public attention to the issue. In 1987, representatives from 43 nations signed the Montreal Protocol. Meanwhile, the halocarbon industry shifted its position and started supporting a protocol to limit CFC production. The reasons for this were in part explained by "Dr. Mostafa Tolba, former head of the UN Environment Programme, who was quoted in the 30 June 1990 edition of The New Scientist, '...the chemical industry supported the Montreal Protocol in 1987 because it set up a worldwide schedule for phasing out CFCs, which [were] no longer protected by patents. This provided companies with an equal opportunity to market new, more profitable compounds.'"[42]
At Montreal, the participants agreed to freeze production of CFCs at 1986 levels and to reduce production by 50% by 1999.[40] After a series of scientific expeditions to the Antarctic produced convincing evidence that the ozone hole was indeed caused by chlorine and bromine from manmade organohalogens, the Montreal Protocol was strengthened at a 1990 meeting in London. The participants agreed to phase out CFCs and halons entirely (aside from a very small amount marked for certain "essential" uses, such as asthma inhalers) by 2000 in non-Article 5 countries and by 2010 in Article 5 (less developed) signatories [43] At a 1992 meeting in Copenhagen, the phase out date was moved up to 1996.[43] At the same meeting, methyl bromide (MeBr), a fumigant used primarily in agricultural production, was added to the list of controlled substances. For all substances controlled under the protocol, phaseout schedules were delayed for less developed ('Article 5(1)') countries, and phaseout in these countries was supported by transfers of expertise, technology, and money from non-Article 5(1) Parties to the Protocol. Additionally, exemptions from the agreed schedules could be applied for under the Essential Use Exemption (EUE) process for substances other than methyl bromide and under the Critical Use Exemption (CUE) process for methyl bromide. See Gareau[44] and DeCanio and Norman[45] for more detail on the exemption processes.
To some extent, CFCs have been replaced by the less damaging hydrochlorofluorocarbons (HCFCs), although concerns remain regarding HCFCs also. In some applications, hydrofluorocarbons (HFCs) have been used to replace CFCs. HFCs, which contain no chlorine or bromine, do not contribute at all to ozone depletion although they are potent greenhouse gases. The best known of these compounds is probably HFC-134a (R-134a), which in the United States has largely replaced CFC-12 (R-12) in automobile air conditioners. In laboratory analytics (a former "essential" use) the ozone depleting substances can be replaced with various other solvents.[46]
Ozone Diplomacy, by Richard Benedick (Harvard University Press, 1991) gives a detailed account of the negotiation process that led to the Montreal Protocol. Pielke and Betsill[dead link] provide an extensive review of early U.S. government responses to the emerging science of ozone depletion by CFCs.
More recently, policy experts have advocated for efforts to link ozone protection efforts to climate protection efforts.[47][48] Many ODS are also greenhouse gases, some significantly more powerful agents of radiative forcing than carbon dioxide over the short and medium term. Policy decisions in one arena affect the costs and effectiveness of environmental improvements in the other.
Prospects of ozone depletion
Since the adoption and strengthening of the Montreal Protocol has led to reductions in the emissions of CFCs, atmospheric concentrations of the most significant compounds have been declining. These substances are being gradually removed from the atmosphere—since peaking in 1994, the Effective Equivalent Chlorine (EECl) level in the atmosphere had dropped about 10% by 2008. It is estimated that by 2015, the Antarctic ozone hole will have reduced by 1 million km² out of 25 (Newman et al., 2004); complete recovery of the Antarctic ozone layer is not expected to occur until the year 2050 or later. Work has suggested that a detectable (and statistically significant) recovery will not occur until around 2024, with ozone levels recovering to 1980 levels by around 2068.[49] The decrease in ozone-depleting chemicals has also been significantly affected by a decrease in bromine-containing chemicals. The data suggest that substantial natural sources exist for atmospheric methyl bromide (CH3Br).[50] The phase-out of CFCs means that nitrous oxide (N2O), which is not covered by the Montreal Protocol, has become the most highly emitted ozone depleting substance and is expected to remain so throughout the 21st century.[51]
When the 2004 ozone hole ended in November 2004, daily minimum stratospheric temperatures in the Antarctic lower stratosphere increased to levels that are too warm for the formation of polar stratospheric clouds (PSCs) about 2 to 3 weeks earlier than in most recent years.[52]
The Arctic winter of 2005 was extremely cold in the stratosphere; PSCs were abundant over many high-latitude areas until dissipated by a big warming event, which started in the upper stratosphere during February and spread throughout the Arctic stratosphere in March. The size of the Arctic area of anomalously low total ozone in 2004–2005 was larger than in any year since 1997. The predominance of anomalously low total ozone values in the Arctic region in the winter of 2004–2005 is attributed to the very low stratospheric temperatures and meteorological conditions favorable for ozone destruction along with the continued presence of ozone destroying chemicals in the stratosphere.[53]
A 2005 IPCC summary of ozone issues concluded that observations and model calculations suggest that the global average amount of ozone depletion has now approximately stabilized. Although considerable variability in ozone is expected from year to year, including in polar regions where depletion is largest, the ozone layer is expected to begin to recover in coming decades due to declining ozone-depleting substance concentrations, assuming full compliance with the Montreal Protocol.[54]
Temperatures during the Arctic winter of 2006 stayed fairly close to the long-term average until late January, with minimum readings frequently cold enough to produce PSCs. During the last week of January, however, a major warming event sent temperatures well above normal — much too warm to support PSCs. By the time temperatures dropped back to near normal in March, the seasonal norm was well above the PSC threshold.[55] Preliminary satellite instrument-generated ozone maps show seasonal ozone buildup slightly below the long-term means for the Northern Hemisphere as a whole, although some high ozone events have occurred.[56] During March 2006, the Arctic stratosphere poleward of 60° North Latitude was free of anomalously low ozone areas except during the three-day period from 17 March to 19 when the total ozone cover fell below 300 DU over part of the North Atlantic region from Greenland to Scandinavia.[57]
The area where total column ozone is less than 220 DU (the accepted definition of the boundary of the ozone hole) was relatively small until around 20 August 2006. Since then the ozone hole area increased rapidly, peaking at 29 million km² 24 September. In October 2006, NASA reported that the year's ozone hole set a new area record with a daily average of 26 million km² between 7 September and 13 October 2006; total ozone thicknesses fell as low as 85 DU on 8 October. The two factors combined, 2006 sees the worst level of depletion in recorded ozone history. The depletion is attributed to the temperatures above the Antarctic reaching the lowest recording since comprehensive records began in 1979.[58][59]
On October 2008 the Ecuadorian Space Agency published a report called HIPERION, a study of the last 28 years data from 10 satellites and dozens of ground instruments around the world among them their own, and found that the UV radiation reaching equatorial latitudes was far greater than expected, climbing in some very populated cities up to 24 UVI, the WHO UV Index standard considers 11 as an extreme index and a great risk to health. The report concluded that the ozone depletion around mid latitudes on the planet is already endangering large populations in this areas. Later, the CONIDA, the Peruvian Space Agency, made its own study, which found almost the same facts as the Ecuadorian study.
The Antarctic ozone hole is expected to continue for decades. Ozone concentrations in the lower stratosphere over Antarctica will increase by 5%–10% by 2020 and return to pre-1980 levels by about 2060–2075, 10–25 years later than predicted in earlier assessments. This is because of revised estimates of atmospheric concentrations of Ozone Depleting Substances — and a larger predicted future usage in developing countries. Another factor which may aggravate ozone depletion is the draw-down of nitrogen oxides from above the stratosphere due to changing wind patterns.[60]
History of the research
The basic physical and chemical processes that lead to the formation of an ozone layer in the Earth's stratosphere were discovered by Sydney Chapman in 1930. These are discussed in the article Ozone-oxygen cycle — briefly, short-wavelength UV radiation splits an oxygen (O2) molecule into two oxygen (O) atoms, which then combine with other oxygen molecules to form ozone. Ozone is removed when an oxygen atom and an ozone molecule "recombine" to form two oxygen molecules, i.e. O + O3 → 2O2. In the 1950s, David Bates and Marcel Nicolet presented evidence that various free radicals, in particular hydroxyl (OH) and nitric oxide (NO), could catalyze this recombination reaction, reducing the overall amount of ozone. These free radicals were known to be present in the stratosphere, and so were regarded as part of the natural balance – it was estimated that in their absence, the ozone layer would be about twice as thick as it currently is.
In 1970 Prof. Paul Crutzen pointed out that emissions of nitrous oxide (N2O), a stable, long-lived gas produced by soil bacteria, from the Earth's surface could affect the amount of nitric oxide (NO) in the stratosphere. Crutzen showed that nitrous oxide lives long enough to reach the stratosphere, where it is converted into NO. Crutzen then noted that increasing use of fertilizers might have led to an increase in nitrous oxide emissions over the natural background, which would in turn result in an increase in the amount of NO in the stratosphere. Thus human activity could have an impact on the stratospheric ozone layer. In the following year, Crutzen and (independently) Harold Johnston suggested that NO emissions from supersonic aircraft, which fly in the lower stratosphere, could also deplete the ozone layer.
The Rowland–Molina hypothesis
In 1974 Frank Sherwood Rowland, Chemistry Professor at the University of California at Irvine, and his postdoctoral associate Mario J. Molina suggested that long-lived organic halogen compounds, such as CFCs, might behave in a similar fashion as Crutzen had proposed for nitrous oxide. James Lovelock (most popularly known as the creator of the Gaia hypothesis) had recently discovered, during a cruise in the South Atlantic in 1971, that almost all of the CFC compounds manufactured since their invention in 1930 were still present in the atmosphere. Molina and Rowland concluded that, like N2O, the CFCs would reach the stratosphere where they would be dissociated by UV light, releasing Cl atoms. (A year earlier, Richard Stolarski and Ralph Cicerone at the University of Michigan had shown that Cl is even more efficient than NO at catalyzing the destruction of ozone. Similar conclusions were reached by Michael McElroy and Steven Wofsy at Harvard University. Neither group, however, had realized that CFCs were a potentially large source of stratospheric chlorine — instead, they had been investigating the possible effects of HCl emissions from the Space Shuttle, which are very much smaller.)
The Rowland–Molina hypothesis was strongly disputed by representatives of the aerosol and halocarbon industries. The Chair of the Board of DuPont was quoted as saying that ozone depletion theory is "a science fiction tale...a load of rubbish...utter nonsense".[42] Robert Abplanalp, the President of Precision Valve Corporation (and inventor of the first practical aerosol spray can valve), wrote to the Chancellor of UC Irvine to complain about Rowland's public statements (Roan, p 56.) Nevertheless, within three years most of the basic assumptions made by Rowland and Molina were confirmed by laboratory measurements and by direct observation in the stratosphere. The concentrations of the source gases (CFCs and related compounds) and the chlorine reservoir species (HCl and ClONO2) were measured throughout the stratosphere, and demonstrated that CFCs were indeed the major source of stratospheric chlorine, and that nearly all of the CFCs emitted would eventually reach the stratosphere. Even more convincing was the measurement, by James G. Anderson and collaborators, of chlorine monoxide (ClO) in the stratosphere. ClO is produced by the reaction of Cl with ozone — its observation thus demonstrated that Cl radicals not only were present in the stratosphere but also were actually involved in destroying ozone. McElroy and Wofsy extended the work of Rowland and Molina by showing that bromine atoms were even more effective catalysts for ozone loss than chlorine atoms and argued that the brominated organic compounds known as halons, widely used in fire extinguishers, were a potentially large source of stratospheric bromine. In 1976 the United States National Academy of Sciences released a report which concluded that the ozone depletion hypothesis was strongly supported by the scientific evidence. Scientists calculated that if CFC production continued to increase at the going rate of 10% per year until 1990 and then remain steady, CFCs would cause a global ozone loss of 5 to 7% by 1995, and a 30 to 50% loss by 2050. In response the United States, Canada and Norway banned the use of CFCs in aerosol spray cans in 1978. However, subsequent research, summarized by the National Academy in reports issued between 1979 and 1984, appeared to show that the earlier estimates of global ozone loss had been too large.[61]
Crutzen, Molina, and Rowland were awarded the 1995 Nobel Prize in Chemistry for their work on stratospheric ozone.
The ozone hole
The discovery of the Antarctic "ozone hole" by British Antarctic Survey scientists Farman, Gardiner and Shanklin (announced in a paper in Nature in May 1985) came as a shock to the scientific community, because the observed decline in polar ozone was far larger than anyone had anticipated.[20] Satellite measurements showing massive depletion of ozone around the south pole were becoming available at the same time. However, these were initially rejected as unreasonable by data quality control algorithms (they were filtered out as errors since the values were unexpectedly low); the ozone hole was detected only in satellite data when the raw data was reprocessed following evidence of ozone depletion in in situ observations. When the software was rerun without the flags, the ozone hole was seen as far back as 1976.[62]
Susan Solomon, an atmospheric chemist at the National Oceanic and Atmospheric Administration (NOAA), proposed that chemical reactions on polar stratospheric clouds (PSCs) in the cold Antarctic stratosphere caused a massive, though localized and seasonal, increase in the amount of chlorine present in active, ozone-destroying forms. The polar stratospheric clouds in Antarctica are only formed when there are very low temperatures, as low as −80 °C, and early spring conditions. In such conditions the ice crystals of the cloud provide a suitable surface for conversion of unreactive chlorine compounds into reactive chlorine compounds which can deplete ozone easily.
Moreover the polar vortex formed over Antarctica is very tight and the reaction which occurs on the surface of the cloud crystals is far different from when it occurs in atmosphere. These conditions have led to ozone hole formation in Antarctica. This hypothesis was decisively confirmed, first by laboratory measurements and subsequently by direct measurements, from the ground and from high-altitude airplanes, of very high concentrations of chlorine monoxide (ClO) in the Antarctic stratosphere.[63]
Alternative hypotheses, which had attributed the ozone hole to variations in solar UV radiation or to changes in atmospheric circulation patterns, were also tested and shown to be untenable.[citation needed]
Meanwhile, analysis of ozone measurements from the worldwide network of ground-based Dobson spectrophotometers led an international panel to conclude that the ozone layer was in fact being depleted, at all latitudes outside of the tropics.[9] These trends were confirmed by satellite measurements. As a consequence, the major halocarbon producing nations agreed to phase out production of CFCs, halons, and related compounds, a process that was completed in 1996.
Since 1981 the United Nations Environment Programme, under the auspices of the World Meteorological Organization, has sponsored a series of technical reports on the Scientific Assessment of Ozone Depletion, based on satellite measurements. The 2007 report showed that the hole in the ozone layer was recovering and the smallest it had been for about a decade.[64] The 2010 report found that "Over the past decade, global ozone and ozone in the Arctic and Antarctic regions is no longer decreasing but is not yet increasing... the ozone layer outside the Polar regions is projected to recover to its pre-1980 levels some time before the middle of this century... In contrast, the springtime ozone hole over the Antarctic is expected to recover much later."[65]
The hole in the Earth’s ozone layer over the South Pole has affected atmospheric circulation in the Southern Hemisphere all the way to the equator. The ozone hole has influenced atmospheric circulation all the way to the tropics and increased rainfall at low, subtropical latitudes in the Southern Hemisphere.
Arctic ozone hole
On March 15, 2011, a record ozone layer loss was observed, with about half of the ozone present over the Arctic having been destroyed.[66][67][68][69] The change was attributed to increasingly cold winters in the Arctic stratosphere at an altitude of approximately 20 km (12 mi), a change associated with global warming in a relationship that is still under investigation.[70] By March 25, the ozone loss had become the largest compared to that observed in all previous winters with the possibility that it would become an ozone hole.[71] This would require that the quantities of ozone to fall below 200 Dobson units, from the 250 recorded over central Siberia.[71] It is predicted that the thinning layer would affect parts of Scandinavia and Eastern Europe on March 30–31.[71]
On 2 October 2011, a study was published in the journal Nature, which said that between December 2010 and March 2011 up to 80% of the ozone in the atmosphere at about 20 kilometres (12 mi) above the surface was destroyed.[72] The level of ozone depletion was severe enough that scientists said it could be compared to the ozone hole that forms over Antarctica every winter.[72] According to the study, "for the first time, sufficient loss occurred to reasonably be described as an Arctic ozone hole."[72] The study analyzed data from the Aura and CALIPSO satellites, and determined that the larger-than-normal ozone loss was due to an unusually long period of cold weather in the Arctic, some 30 days more than typical, which allowed for more ozone-destroying chlorine compounds to be created.[73] According to Lamont Poole, a co-author of the study, cloud and aerosol particles on which the chlorine compounds are found "were abundant in the Arctic until mid March 2011—much later than usual—with average amounts at some altitudes similar to those observed in the Antarctic, and dramatically larger than the near-zero values seen in March in most Arctic winters."[73]
Tibet ozone hole
As winters that are colder are more affected, at times there is an ozone hole over Tibet. In 2006, a 2.5 million square kilometer ozone hole was detected over Tibet.[74] Also again in 2011 an ozone hole appeared over mountainous regions of Tibet, Xinjiang, Qinghai and the Hindu Kush, along with an unprecedented hole over the Arctic, though the Tibet one is far less intense than the ones over the Arctic or Antarctic.[75]
Ozone depletion and global warming
There are five areas of linkage between ozone depletion and global warming:
- The same CO2 radiative forcing that produces global warming is expected to cool the stratosphere.[76] This cooling, in turn, is expected to produce a relative increase in ozone (O3) depletion in polar area and the frequency of ozone holes.[77]
- Conversely, ozone depletion represents a radiative forcing of the climate system. There are two opposing effects: Reduced ozone causes the stratosphere to absorb less solar radiation, thus cooling the stratosphere while warming the troposphere; the resulting colder stratosphere emits less long-wave radiation downward, thus cooling the troposphere. Overall, the cooling dominates; the IPCC concludes that "observed stratospheric O3 losses over the past two decades have caused a negative forcing of the surface-troposphere system"[10] of about −0.15 ± 0.10 watts per square meter (W/m²).[78]
- One of the strongest predictions of the greenhouse effect is that the stratosphere will cool.[76] Although this cooling has been observed, it is not trivial to separate the effects of changes in the concentration of greenhouse gases and ozone depletion since both will lead to cooling. However, this can be done by numerical stratospheric modeling. Results from the National Oceanic and Atmospheric Administration's Geophysical Fluid Dynamics Laboratory show that above 20 km (12 mi), the greenhouse gases dominate the cooling.[79]
- As noted under 'Public Policy', ozone depleting chemicals are also often greenhouse gases. The increases in concentrations of these chemicals have produced 0.34 ± 0.03 W/m² of radiative forcing, corresponding to about 14% of the total radiative forcing from increases in the concentrations of well-mixed greenhouse gases.[78]
- The long term modeling of the process, its measurement, study, design of theories and testing take decades to document, gain wide acceptance, and ultimately become the dominant paradigm. Several theories about the destruction of ozone were hypothesized in the 1980s, published in the late 1990s, and are currently being investigated. Dr Drew Schindell, and Dr Paul Newman, NASA Goddard, proposed a theory in the late 1990s, using a SGI Origin 2000 supercomputer, that modeled ozone destruction, accounted for 78% of the ozone destroyed. Further refinement of that model accounted for 89% of the ozone destroyed, but pushed back the estimated recovery of the ozone hole from 75 years to 150 years. (An important part of that model is the lack of stratospheric flight due to depletion of fossil fuels.)
Misconceptions about ozone depletion
- CFCs are "too heavy" to reach the stratosphere
Since CFC molecules are heavier than air (nitrogen or oxygen), it is commonly believed that the CFC molecules cannot reach the stratosphere in significant amount.[80] But atmospheric gases are not sorted by weight; the forces of wind can fully mix the gases in the atmosphere. The CFCs are evenly distributed throughout the turbosphere and reach the upper atmosphere.[81]
- Man-made chlorine is insignificant compared to natural sources
Another misconception is that "it is generally accepted that natural sources of tropospheric chlorine are four to five times larger than man-made one". While strictly true, tropospheric chlorine is irrelevant; it is stratospheric chlorine that affects ozone depletion. Chlorine from ocean spray is soluble and thus is washed by rainfall before it reaches the stratosphere. CFCs, in contrast, are insoluble and long-lived, allowing them to reach the stratosphere. In the lower atmosphere, there is much more chlorine from CFCs and related haloalkanes than there is in HCl from salt spray, and in the stratosphere halocarbons are dominant .[82] Only methyl chloride which is one of these halocarbons has a mainly natural source ,[83] and it is responsible for about 20 percent of the chlorine in the stratosphere; the remaining 80% comes from man made sources.
Very violent volcanic eruptions can inject HCl into the stratosphere, but researchers[84] have shown that the contribution is not significant compared to that from CFCs. A similar erroneous assertion is that soluble halogen compounds from the volcanic plume of Mount Erebus on Ross Island, Antarctica are a major contributor to the Antarctic ozone hole.[84]
- An ozone hole was first observed in 1956
G.M.B. Dobson (Exploring the Atmosphere, 2nd Edition, Oxford, 1968) mentioned that when springtime ozone levels over Halley Bay were first measured in 1956, he was surprised to find that they were ~320 DU, about 150 DU below spring levels, ~450 DU, in the Arctic. These, however, were at this time the known normal climatological values because no other Antarctic ozone data were available. What Dobson describes is essentially the baseline from which the ozone hole is measured: actual ozone hole values are in the 150–100 DU range.
The discrepancy between the Arctic and Antarctic noted by Dobson was primarily a matter of timing: during the Arctic spring ozone levels rose smoothly, peaking in April, whereas in the Antarctic they stayed approximately constant during early spring, rising abruptly in November when the polar vortex broke down.
The behavior seen in the Antarctic ozone hole is completely different. Instead of staying constant, early springtime ozone levels suddenly drop from their already low winter values, by as much as 50%, and normal values are not reached again until December.[85]
- The ozone hole should be above the sources of CFCs
Some people thought that the ozone hole should be above the sources of CFCs. However, CFCs are well mixed globally in the troposphere and the stratosphere. The reason for occurrence of the ozone hole above Antarctica is not because there are more CFCs concentrated but because the low temperatures help form polar stratospheric clouds.[86] In fact, there are findings of significant and localized "ozone holes" above other parts of the earth.[87]
- The "ozone hole" is a hole in the ozone layer
There is a common misconception that the “ozone hole” is really a hole in the ozone layer. When the "ozone hole" occurs, the ozone in the lower stratosphere is destroyed. The upper stratosphere is less affected, so that the amount of ozone over the continent decreases by 50 percent or even more. The ozone does not disappear through the layer, nor is there a uniform 'thinning' of the ozone layer. It is a "hole" which is a depression, not in the sense of "a hole in the windshield."
ODS requirements in the marine industry
IMO has amended MARPOL Annex VI Regulation 12 regarding ozone depleting substances.
As from July 1, 2010, all vessels where MARPOL Annex VI is applicable should have a list of equipment using ozone depleting substances. The list should include name of ODS, type and location of equipment, quantity in kg and date. All changes since that date should be recorded in a ODS Record book on board recording all intended or unintended releases to the atmosphere. Furthermore, new ODS supply or landing to shore facilities should be recorded as well.
World Ozone Day
In 1994, the United Nations General Assembly voted to designate September 16 as "World Ozone Day", to commemorate the signing of the Montreal Protocol on that date in 1987.
See also
- Atmospheric window
- Health effects of sun exposure
- Vitamin D
- Ultraviolet
References
- ^ "Part III. The Science of the Ozone Hole". http://www.atm.ch.cam.ac.uk/tour/part3.html. Retrieved 2007-03-05.
- ^ Andino, Jean M. (21 October 1999). "Chlorofluorocarbons (CFCs) are heavier than air, so how do scientists suppose that these chemicals reach the altitude of the ozone layer to adversely affect it?". Sci. Am.. http://www.sciam.com/article.cfm?id=chlorofluorocarbons-cfcs.
- ^ Dobson, R. (2005). "Ozone depletion will bring big rise in number of cataracts". BMJ 331 (7528): 1292–1295. doi:10.1136/bmj.331.7528.1292-d. PMC 1298891. PMID 0. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1298891.
- ^ Newman, Paul A.. "Chapter 5: Stratospheric Photochemistry Section 4.2.8 ClX catalytic reactions". In Todaro, Richard M.. Stratospheric ozone: an electronic textbook. NASA Goddard Space Flight Center Atmospheric Chemistry and Dynamics Branch. http://www.ccpo.odu.edu/SEES/ozone/class/Chap_5/index.htm.[dead link]
- ^ "Stratospheric Ozone Depletion by Chlorofluorocarbons (Nobel Lecture)—Encyclopedia of Earth". Eoearth.org. http://www.eoearth.org/article/Stratospheric_Ozone_Depletion_by_Chlorofluorocarbons_(Nobel_Lecture). Retrieved 2011-03-28.
- ^ "The Ozone Hole Tour: Part II. Recent Ozone Depletion". Atm.ch.cam.ac.uk. http://www.atm.ch.cam.ac.uk/tour/part2.html. Retrieved 2011-03-28.
- ^ PWMU. "World Meteorological Organization (WMO)". Wmo.ch. http://www.wmo.ch/web/arep/reports/o3_assess_rep_2002_front_page.html. Retrieved 2011-03-28.[dead link]
- ^ U.S. EPA: Ozone Depletion[dead link]
- ^ a b "Myth: Ozone Depletion Occurs Only In Antarctica | Science | Ozone Layer Protection | US EPA". Epa.gov. 2006-06-28. http://www.epa.gov/ozone/science/myths/glob_dep.html. Retrieved 2011-03-28.
- ^ a b "Climate Change 2001: Working Group I: The Scientific Basis". Intergovernmental Panel on Climate Change Work Group I. 2001. pp. Chapter 6.4 Stratospheric Ozone. http://www.grida.no/climate/ipcc_tar/wg1/223.htm.
- ^ [1][dead link]
- ^ Newman, P.A., Daniel, J.S., Waugh, D.W., Nash, E.R. (2007). "A new formulation of equivalent effective stratospheric chlorine (EESC)". Atmos. Chem. Phys. 7 (17): 4537–52. doi:10.5194/acp-7-4537-2007. http://www.atmos-chem-phys.net/7/4537/2007/acp-7-4537-2007.html.
- ^ "chlorofluorocarbons". Encyclopedia.com. http://www.encyclopedia.com/doc/1E1-chlorofl.html. Retrieved 2011-03-28.
- ^ Nash, Eric; Newman, Paul (September 19, 2001). "NASA Confirms Arctic Ozone Depletion Trigger". Image of the Day. NASA. http://earthobservatory.nasa.gov/IOTD/view.php?id=1771. Retrieved April 16, 2011.
- ^ Sparling, Brien (June 26, 2001). "Antarctic Ozone Hole". NASA Advanced Supercomputing Department. http://www.nas.nasa.gov/About/Education/Ozone/antarctic.html. Retrieved April 16, 2011.
- ^ Parson, Robert (December 16, 1997). "Antarctic ozone-depletion FAQ, section 7". Faqs.org. http://www.faqs.org/faqs/ozone-depletion/antarctic. Retrieved April 16, 2011.
- ^ Toon, Owen B.; Turco, Richard P. (June 1991). "Polar Stratospheric Clouds and Ozone Depletion". Scientific American 264 (6): 68–74. Bibcode 1991SciAm.264...68T. doi:10.1038/scientificamerican0691-68. http://www.atmos.washington.edu/~davidc/ATMS211/articles_optional/Toon_Turco91_ozone.pdf. Retrieved April 16, 2011.
- ^ "Ozone Facts: What is the Ozone Hole?". Ozone Hole Watch. NASA. November 18, 2009. http://ozonewatch.gsfc.nasa.gov/facts/hole.html. Retrieved April 16, 2011.
- ^ Rowland, Frank Sherwood (29 May 2006). "Stratospheric ozone depletion". Phil. Trans. R. Soc. B 361 (1469): 769–790. doi:10.1098/rstb.2005.1783. PMC 1609402. PMID 16627294. http://rstb.royalsocietypublishing.org/content/361/1469/769.full. "4. Free radical reactions for ozone removal: Reaction 4.1"
- ^ a b Stephen C. Zehr (November 1994). "Accounting for the Ozone Hole: Scientific Representations of an Anomaly and Prior Incorrect Claims in Public Settings". The Sociological Quarterly 35 (4): 603–19. doi:10.1111/j.1533-8525.1994.tb00419.x. JSTOR 4121521.
- ^ "Climate Change 2001: Working Group I: The Scientific Basis". Intergovernmental Panel on Climate Change Work Group I. 2001. pp. Chapter 9.3.2 Patterns of Future Climate Change. http://www.grida.no/publications/other/ipcc%5Ftar/?src=/climate/ipcc_tar/wg1/351.htm.
- ^ Muir, Patricia (March 6, 2008). "Stratospheric Ozone Depletion". Oregon State University. http://people.oregonstate.edu/~muirp/stratozo.htm. Retrieved April 16, 2011.
- ^ "UV & Ozone". National Institute of Water & Atmospheric Research, NZ. http://www.niwa.co.nz/services/uvozone.
- ^ "Ozone Hole Over City for First Time – ABC News". Abcnews.go.com. http://abcnews.go.com/Technology/story?id=119899&page=1. Retrieved 2011-03-28.
- ^ "Bad Nearby | Ozone – Good Up High Bad Nearby | Air Quality Planning & Standards | Air & Radiation | US EPA". Epa.gov. 2006-06-28. http://www.epa.gov/oar/oaqps/gooduphigh/bad.html#6. Retrieved 2011-03-28.
- ^ a b Frank R. de Gruijl (Summer 1995). "Impacts of a Projected Depletion of the Ozone Layer". Consequences 1 (2). http://www.gcrio.org/CONSEQUENCES/summer95/impacts.html.
- ^ Setlow RB, Grist E, Thompson K, Woodhead AD (July 1993). "Wavelengths effective in induction of malignant melanoma". Proc. Natl. Acad. Sci. U.S.A. 90 (14): 6666–70. Bibcode 1993PNAS...90.6666S. doi:10.1073/pnas.90.14.6666. PMC 46993. PMID 8341684. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=46993.
- ^ Fears TR, Bird CC, Guerry D, et al. (July 2002). "Average midrange ultraviolet radiation flux and time outdoors predict melanoma risk". Cancer Res. 62 (14): 3992–6. PMID 12124332. http://cancerres.aacrjournals.org/cgi/pmidlookup?view=long&pmid=12124332.
- ^ Abarca JF, Casiccia CC (December 2002). "Skin cancer and ultraviolet-B radiation under the Antarctic ozone hole: southern Chile, 1987–2000". Photodermatol Photoimmunol Photomed 18 (6): 294–302. doi:10.1034/j.1600-0781.2002.02782.x. PMID 12535025. http://www.blackwell-synergy.com/links/doi/10.1034/j.1600-0781.2002.02782.x/full/.
- ^ West SK, Duncan DD, Muñoz B, et al. (August 1998). "Sunlight exposure and risk of lens opacities in a population-based study: the Salisbury Eye Evaluation project". JAMA 280 (8): 714–8. doi:10.1001/jama.280.8.714. PMID 9728643. http://jama.ama-assn.org/cgi/content/full/280/8/714.
- ^ Cruickshanks KJ, Klein BE, Klein R (December 1992). "Ultraviolet light exposure and lens opacities: the Beaver Dam Eye Study". Am J Public Health 82 (12): 1658–62. doi:10.2105/AJPH.82.12.1658. PMC 1694542. PMID 1456342. http://www.ajph.org/cgi/pmidlookup?view=long&pmid=1456342.
- ^ West SK, Muñoz B, Schein OD, Duncan DD, Rubin GS (December 1998). "Racial differences in lens opacities: the Salisbury Eye Evaluation (SEE) project". Am. J. Epidemiol. 148 (11): 1033–9. PMID 9850124. http://aje.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=9850124.
- ^ Leske MC, Connell AM, Wu SY, Hyman L, Schachat A (January 1997). "Prevalence of lens opacities in the Barbados Eye Study". Arch. Ophthalmol. 115 (1): 105–11. PMID 9006434. http://archopht.ama-assn.org/cgi/pmidlookup?view=long&pmid=9006434.
- ^ Melamed ML, Michos ED, Post W, Astor B (August 2008). "25-hydroxyl Vitamin D Levels and the Risk of Mortality in the General Population". Arch. Intern. Med. 168 (15): 1629–37. doi:10.1001/archinte.168.15.1629. PMC 2677029. PMID 18695076. http://archinte.ama-assn.org/cgi/pmidlookup?view=long&pmid=18695076.
- ^ Vieth R (May 1999). "Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety". Am. J. Clin. Nutr. 69 (5): 842–56. PMID 10232622. http://www.ajcn.org/cgi/pmidlookup?view=long&pmid=10232622.
- ^ "Sunburned whales: Troubling environment news of the week". Voices.washingtonpost.com. 2010-11-11. http://voices.washingtonpost.com/blog-post/2010/11/sunburned_whales_bad_environme.html. Retrieved 2011-03-28.
- ^ Abbie Thomas (2010-11-10). "Whales showing more sun damage". 23;-102: Abc.net.au. http://www.abc.net.au/science/articles/2010/11/10/3062051.htm. Retrieved 2011-03-28.
- ^ R. P. Sinha; S. C. Singh and D.-P. Häder (1999). "Photoecophysiology of cyanobacteria". Journal of Photochemistry and Photobiology 3: 91–101.
- ^ National Academy of Sciences (1976). Halocarbons, effects on stratospheric ozone. Washington, DC. http://books.google.com/?id=a2YrAAAAYAAJ&dq=Halocarbons:+Effects+on+Stratospheric+Ozone.
- ^ a b c d Morrisette, Peter M. (1989). "The Evolution of Policy Responses to Stratospheric Ozone Depletion". Natural Resources Journal 29: 793–820. http://www.ciesin.org/docs/003-006/003-006.html. Retrieved 2010-04-20.
- ^ http://www.ghgregistries.ca/registry/out/C650-DUPONT-PLN.PDF
- ^ a b "Du Pont: A case study in the 3d corporate strategy". Archive.greenpeace.org. http://archive.greenpeace.org/ozone/greenfreeze/moral97/6dupont.html. Retrieved 2011-03-28.
- ^ a b "Amendments to the Montreal Protocol | Ozone Layer Protection | US EPA". Epa.gov. 2006-06-28. http://www.epa.gov/ozone/intpol/history.html. Retrieved 2011-03-28.
- ^ Gareau BJ (2010). "A critical review of the successful CFC phase-out versus the delayed methyl bromide phase-out in the Montreal Protocol". International Environmental Agreements-Politics Law and Economics 10 (3): 209–231. doi:10.1007/s10784-010-9120-z.
- ^ DeCanio SJ, Norman CS (July 2005). "Economics of the 'Critical use' of Methyl Bromide under the Montreal Protocol". Contemporary Economic Policy 23 (3): 376–393. doi:10.1093/cep/byi028.
- ^ "Use of Ozone Depleting Substances in Laboratories. TemaNord 516/2003". Norden.org. 2003-01-01. http://www.norden.org/pub/ebook/2003-516.pdf. Retrieved 2011-03-28.
- ^ Mario Molina, Durwood Zaelke, K. Madhava Sarma, Stephen O. Andersen, Veerabhadran Ramanathan, Donald Kaniaru (2009). "Reducing abrupt climate change risk using the Montreal Protocol and other regulatory actions to complement cuts in CO2 emissions". Proc. Natl. Acad. Sci. U.S.A. 106 (49): 20616–21. Bibcode 2009PNAS..10620616M. doi:10.1073/pnas.0902568106. PMC 2791591. PMID 19822751. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2791591.
- ^ Norman CS, DeCanio SJ, Fan L (May 2008). "The Montreal Protocol at 20: Ongoing opportunities for integration with climate protection". Global Environmental Change 18 (2): 330–340. doi:10.1016/j.gloenvcha.2008.03.003.
- ^ Newman, P. A., Nash, E. R., Kawa, S. R., Montzka, S. A. and Schauffler, S. M (2006). "When will the Antarctic ozone hole recover?". Geophysical Research Letters 33 (12): L12814. Bibcode 2006GeoRL..3312814N. doi:10.1029/2005GL025232.
- ^ PWMU. "World Meteorological Organization (WMO)". Wmo.ch. http://www.wmo.ch/web/arep/reports/ozone_2002/06_chapter1.pdf. Retrieved 2011-04-06.[dead link]
- ^ "NOAA Study Shows Nitrous Oxide Now Top Ozone-Depleting Emission". Noaanews.noaa.gov. 2009-08-27. http://www.noaanews.noaa.gov/stories2009/20090827_ozone.html. Retrieved 2011-04-06.
- ^ PWMU. "World Meteorological Organization (WMO)". Wmo.ch. http://www.wmo.ch/web/arep/04/bulletin_7_2004.pdf. Retrieved 2011-04-06.[dead link]
- ^ "CPC—Stratosphere: Winter Bulletins". Cpc.ncep.noaa.gov. 2005-05-18. http://www.cpc.ncep.noaa.gov/products/stratosphere/winter_bulletins/nh_04-05/index.html. Retrieved 2011-04-06.
- ^ "Summary for Policymakers". IPCC/TEAP special report on safeguarding the ozone layer and the global climate system: issues related to hydrofluorocarbons and perfluorocarbons. Cambridge: Published for the Intergovernmental Panel on Climate Change [by] Cambridge University Press. 2005. ISBN 0-521-86336-8. http://www.ipcc.ch/pdf/special-reports/sroc/sroc_spm.pdf.
- ^ "Available Annual NCEP data". Code916.gsfc.nasa.gov. http://code916.gsfc.nasa.gov/Data_services/met/ann_data.html. Retrieved 2011-04-06.
- ^ "Select ozone maps, individual sources". Es-ee.tor.ec.gc.ca. 1998-06-01. http://es-ee.tor.ec.gc.ca/cgi-bin/dailyMaps. Retrieved 2011-04-06.
- ^ "Index of /products/stratosphere/sbuv2to/archive/nh". Cpc.ncep.noaa.gov. http://www.cpc.ncep.noaa.gov/products/stratosphere/sbuv2to/archive/nh. Retrieved 2011-04-06.
- ^ "Ozone Hole Watch". Ozonewatch.gsfc.nasa.gov. http://ozonewatch.gsfc.nasa.gov. Retrieved 2011-04-06.
- ^ "Ozone layer hits new depletion record". The Register. 2006-10-03. http://www.theregister.co.uk/2006/10/03/ozone_depletion.
- ^ CNW Group | CANADIAN SPACE AGENCY | Canada's SCISAT satellite explains 2006 ozone-layer depletion[dead link]
- ^ Causes and Effects of Stratospheric Ozone Reduction: An Update. National Academy of Sciences. (1982 and 1983). http://www.nap.edu/openbook.php?isbn=0309032482.
- ^ Ozone Depletion, History and politics accessed 18 November 2007.
- ^ P. M. Solomon, B. Connor, R. L. de Zafra, A. Parrish, J. Barrett, M. Jaramillo (July 1987). "High concentrations of chlorine monoxide at low altitudes in the Antarctic spring stratosphere: secular variation". Nature 328 (6129): 411–3. doi:10.1038/328411a0. http://www.nature.com/nature/journal/v328/n6129/abs/328411a0.html.
- ^ "Ozone hole closing up, research shows". ABC News (Australian Broadcasting Commission). 2007-11-16. http://abc.net.au/news/stories/2007/11/16/2092527.htm.
- ^ "New report highlights two-way link between ozone layer and climate change". UNEP News Center. 2010-11-16. http://www.unep.org/Documents.Multilingual/Default.asp?DocumentID=647&ArticleID=6751&l=en&t=long.
- ^ Arctic on the verge of record ozone loss - Arctic-wide measurements verify rapid depletion in recent days
- ^ Christine Dell'Amore (2011-03-22). "First North Pole Ozone Hole Forming?". News.nationalgeographic.com. http://news.nationalgeographic.com/news/2011/03/110321-ozone-layer-hole-arctic-north-pole-science-environment-uv-sunscreen/. Retrieved 2011-04-06.
- ^ "Arctic On Verge Of Record Ozone Loss". Eurasia Review - Eurasiareview.com. 2011-03-25. http://www.eurasiareview.com/arctic-on-verge-of-record-ozone-loss-25032011/. Retrieved 2011-04-06.
- ^ "The Arctic Ozone Sieve: More Global Weirding? : Dean's Corner". Scienceblogs.com. 2011-03-25. http://scienceblogs.com/deanscorner/2011/03/the_arctic_ozone_sieve_more_gl.php. Retrieved 2011-04-06.
- ^ "Arctic on the verge of record ozone loss". Sciencedaily.com. 2011-03-14. http://www.sciencedaily.com/releases/2011/03/110314100835.htm. Retrieved 2011-04-06.
- ^ a b c "Developing ozone hole approaches Europe". EurActiv. http://www.euractiv.com/en/climate-environment/developing-ozone-hole-approaches-europe-news-503504. Retrieved 2011-04-06.
- ^ a b c "Arctic ozone loss at record level". BBC News Online. 2 October 2011. Archived from the original on 2 October 2011. http://www.webcitation.org/6297yWsLm. Retrieved 3 October 2011.
- ^ a b "Unprecedented Arctic Ozone Loss in 2011, Says NASA-Led Study". MarketWatch. 2 October 2011. Archived from the original on 2 October 2011. http://www.webcitation.org/6297sB16L. Retrieved 3 October 2011.
- ^ "Earth news: Chinese Scientists Find New Ozone Hole Over Tibet". Elainemeinelsupkis.typepad.com. 2006-05-04. http://elainemeinelsupkis.typepad.com/earth_news/2006/05/chinese_scienti.html. Retrieved 2011-04-06.
- ^ Schiermeier, Quirin (1999-02-22). "The Great Beyond: Arctic ozone hole causes concern". Blogs.nature.com. http://blogs.nature.com/news/thegreatbeyond/2011/04/arctic_ozone_hole_causes_conce.html. Retrieved 2011-04-06.
- ^ a b Hegerl, Gabriele C.; et al.. "Understanding and Attributing Climate Change" (PDF). Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Intergovernmental Panel on Climate Change. p. 675. http://www.ipcc.ch/pdf/assessment-report/ar4/wg1/ar4-wg1-chapter9.pdf. Retrieved 2008-02-01.
- ^ http://earthwatch.unep.net/emergingissues/atmosphere/ozonedepletion.php
- ^ a b (PDF) IPCC/TEAP Special Report on Safeguarding the Ozone Layer and the Global Climate System: Issues Related to Hydrofluorocarbons and Perfluorocarbons (summary for policy makers). International Panel on Climate Change and Technology and Economic Assessment Panel. 2005. Archived from the original on February 21, 2007. http://web.archive.org/web/20070221055911/http://www.ipcc.ch/press/SPM.pdf. Retrieved 2007-03-04.
- ^ "The Relative Roles of Ozone and Other Greenhouse Gases in Climate Change in the Stratosphere". Geophysical Fluid Dynamics Laboratory. 2007-02-29. http://www.gfdl.noaa.gov/aboutus/milestones/ozone.html. Retrieved 2007-03-04.
- ^ Amy Silverman (1995-05-04). "FREON EASY". Phoenix News. http://www.phoenixnewtimes.com/1995-05-04/news/freon-easy/full. Retrieved 2011-04-06.
- ^ FAQ, part I, section 1.3.
- ^ ozone-depletion FAQ, Part II, section 4.3
- ^ Y. Yokouchi; Y. Noijiri; L. A. Barrie; D. Toom-Sauntry; T. Machida; Y. Inuzuka; H. Akimoto; Li, HJ et al. (2000-01-20). "A strong source of methyl chloride to the atmosphere from tropical coastal land". Nature 403 (6767): 295–8. Bibcode 2000Natur.403..295Y. doi:10.1038/35002049. PMID 10659845. http://www.nature.com/nature/journal/v403/n6767/full/403295a0.html.
- ^ a b ozone-depletion FAQ, Part II, section 4.4
- ^ ozone-depletion FAQ, Part III, section 6
- ^ "ozone-depletion FAQ, Antarctic". Faqs.org. http://www.faqs.org/faqs/ozone-depletion/antarctic. Retrieved 2011-04-06.
- ^ "Ozone hole: Definition". Answers.com. http://www.answers.com/topic/ozone-depletion. Retrieved 2011-04-06.
Nontechnical books
- Dray, Philip; Cagin, Seth (1993). Between earth and sky: how CFCs changed our world and endangered the ozone layer. New York: Pantheon Books. ISBN 0-679-42052-5.
- Roan, Sharon (1989). Ozone crisis: The 15-year evolution of a sudden global emergency. New York: Wiley. ISBN 0-471-52823-4.
- Schiff, Harold; Dotto, Lydia; (1978). The Ozone war. Garden City, N.Y: Doubleday. ISBN 0-385-12927-0.
Books on public policy issues
- Benedick, Richard Elliot (1991). Ozone diplomacy: New directions in safeguarding the planet. Cambridge: Harvard University Press. ISBN 0-674-65001-8. (Ambassador Benedick was the Chief U.S. Negotiator at the meetings that resulted in the Montreal Protocol.)
- Litfin, Karen (1994). Ozone discourses: Science and politics in global environmental cooperation. New York: Columbia University Press. ISBN 0-231-08137-5.
Research articles
- Newman, P. A., Kawa, S. R. and Nash, E. R. (2004). "On the size of the Antarctic ozone hole?". Geophysical Research Letters 31 (21): L12814. Bibcode 2004GeoRL..3121104N. doi:10.1029/2004GL020596.
- E. C. Weatherhead, S. B. Andersen (2006). "The search for signs of recovery of the ozone layer". Nature 441 (7089): 39–45. Bibcode 2006Natur.441...39W. doi:10.1038/nature04746. PMID 16672963.
External links
- Ozone layer at the Open Directory Project
- UN Chronicle Unlayering of the Ozone: An Earth Sans Sunscreen
- NOAA/ESRL Ozone Depletion
- NOAA Ozone Depleting Gas Index
- The Ozone Hole
Pollution Air pollution Acid rain · Air quality (Indoor) · Atmospheric dispersion modeling · Chlorofluorocarbon · Global dimming · Global distillation · Global warming · Ozone depletion · Particulates · SmogWater pollution Environmental impact of pharmaceuticals and personal care products · Environmental impact of shipping · Environmental monitoring · Eutrophication · Freshwater environmental quality parameters · Hypoxia · Marine debris · Marine pollution · Ocean acidification · Oil spill · Surface runoff · Thermal pollution · Urban runoff · Wastewater · Water quality · Water stagnation · Waterborne diseasesSoil contamination Bioremediation · Phytoremediation · Electrical resistance heating · Herbicide · Pesticide · Soil Guideline Values (SGVs)Radioactive contamination Actinides in the environment · Environmental radioactivity · Fission product · Nuclear fallout · Plutonium in the environment · Radiation poisoning · Radium in the environment · Uranium in the environmentOther types of pollution Inter-government treaties Major organizations DEFRA · Environment Agency (England and Wales) · Scottish Environment Protection Agency (Scotland) · U.S. EPA · EEA · GreenpeaceCategories:- Ozone depletion
- Environmental issues
- Millennium Development Goals
Wikimedia Foundation. 2010.