- Cyclobutadieneiron tricarbonyl
-
Cyclobutadieneiron tricarbonyl 
Properties Molecular formula (C4H4)Fe(CO)3  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Cyclobutadieneiron tricarbonyl or (C4H4)Fe(CO)3 is an organometallic complex of cyclobutadiene and an iron metal carbonyl. The chemical compound is used in organic chemistry as a precursor for cyclobutadiene. It was first prepared in 1965 by Rowland Pettit starting from cyclooctatetraene [1] [2] [3] :
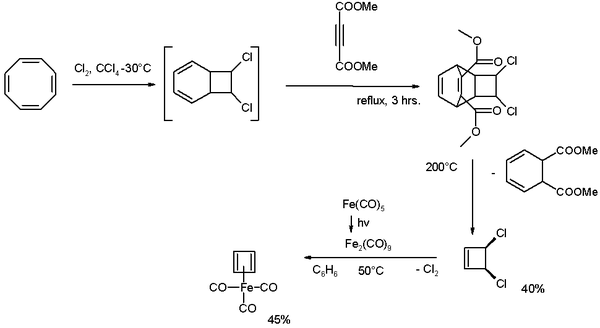
Cyclooctatetraene is chlorinated to the [4.2.0]-bicyclic compound which reacts further with the alkyne dimethyl acetylenedicarboxylate in a Diels-Alder reaction followed by a reverse-DA reaction by pyrolysis at 200°C releasing cis-dichlorocyclobutene.
This compound reacts with di-iron nonacarbonyl (obtained from photolysis of iron pentacarbonyl) to Cyclobutadieneiron tricarbonyl.
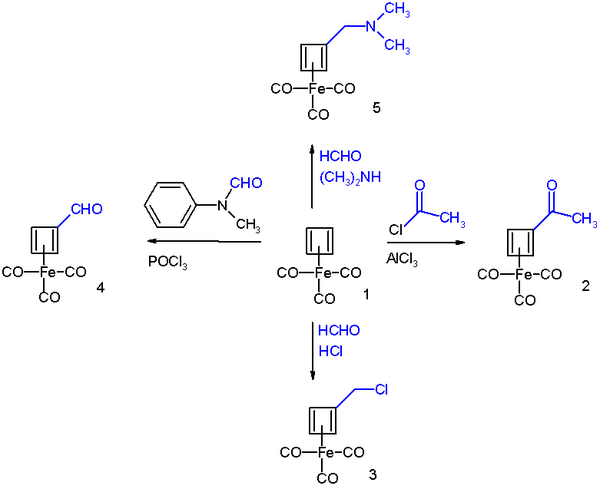
Cyclobutadieneiron tricarbonyl displays aromaticity as evidenced by some of its reactions which can be classified as electrophilic aromatic substitution [4] :
It reacts in Friedel-Crafts acylation with acetyl chloride and aluminium chloride to the acyl derivative 2, with formaldehyde and hydrochloric acid to the chloromethyl derivative 3, in a Vilsmeier-Haack reaction with N-methylformanilide and phosphorus oxychloride to the formyl 4 and in a Mannich reaction to amine derivative 4
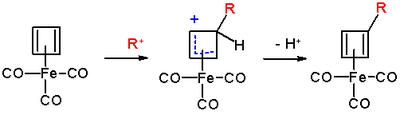
The reaction mechanism is identical to that of EAS:
References
- ^ Cyclobutadiene- and Benzocyclobutadiene-Iron Tricarbonyl Complexes G. F. Emerson, L. Watts, R. Pettit; J. Am. Chem. Soc.; 1965; 87(1); 131-133. First Page
- ^ Cis-dichlorocyclobutene , Organic Syntheses, Coll. Vol. 6, p.422 (1988); Vol. 50, p.36 (1970) Article.
- ^ Iron, tricarbonyl (η4-1,3-cyclobutadiene)- R. Pettit and J. Henery Organic Syntheses, Coll. Vol. 6, p.310 (1988); Vol. 50, p.21 (1970) Link
- ^ Cyclobutadieneiron Tricarbonyl. A New Aromatic System J. D. Fitzpatrick, L. Watts, G. F. Emerson, R. Pettit J. Am. Chem. Soc.; 1965; 87(14); 3254-3255 Abstract
Further reading
- P. D. Harvey, W. P. Schaefer, H. B. Gray, D. F. R. Gilson, I. S. Butler (1988). "Structure of tricarbonyl(η4-cyclobutadienyl)iron(0) at −45 °C". Inorg. Chem. 27 (1): 57–59. doi:10.1021/ic00274a013.
Categories:- Carbonyl complexes
- Organoiron compounds
Wikimedia Foundation. 2010.