- Tetracycline
-
This article is about the specific antibiotic called tetracycline. For other uses, see tetracycline antibiotics.
Tetracycline 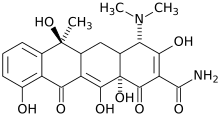
Systematic (IUPAC) name (4S,6S,12aS)-4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxonaphthacene-2-carboxamide
OR
(4S,6S,12aS)-4-(dimethylamino)-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamideClinical data Trade names Sumycin AHFS/Drugs.com monograph MedlinePlus a682098 Licence data US FDA:link Pregnancy cat. D(AU) D(US) Legal status ℞ Prescription only Routes oral, topical (skin & eye), im, iv Pharmacokinetic data Bioavailability 60-80% Oral, while fasting
<40% IntramuscularMetabolism Not metabolised Half-life 6-11 hours Excretion Fecal and Renal Identifiers CAS number 60-54-8 
64-75-5 (hydrochloride)ATC code A01AB13 D06AA04 J01AA07 S01AA09 S02AA08 S03AA02 QG01AA90 QG51AA02 QJ51AA07 PubChem CID 643969 DrugBank DB00759 ChemSpider 10257122 
UNII F8VB5M810T 
KEGG D00201 
ChEBI CHEBI:27902 
ChEMBL CHEMBL1440 
Chemical data Formula C22H24N2O8 Mol. mass 444.435 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Tetracycline (INN) (
 /ˌtɛtrəˈsaɪkliːn/) is a broad-spectrum polyketide antibiotic produced by the Streptomyces genus of Actinobacteria, indicated for use against many bacterial infections. It is a protein synthesis inhibitor. It is commonly used to treat acne today, and, more recently, rosacea, and is historically important in reducing the number of deaths from cholera. Tetracycline is marketed under the brand names Sumycin, Tetracyn, and Panmycin, among others. Actisite is a thread-like fiber formulation, used in dental applications. It is also used to produce several semisynthetic derivatives, which together are known as the tetracycline antibiotics. The term tetracycline is also used to denote the 4-ring system of this compound; tetracyclines are related substances that contain the same 4-ring system.
/ˌtɛtrəˈsaɪkliːn/) is a broad-spectrum polyketide antibiotic produced by the Streptomyces genus of Actinobacteria, indicated for use against many bacterial infections. It is a protein synthesis inhibitor. It is commonly used to treat acne today, and, more recently, rosacea, and is historically important in reducing the number of deaths from cholera. Tetracycline is marketed under the brand names Sumycin, Tetracyn, and Panmycin, among others. Actisite is a thread-like fiber formulation, used in dental applications. It is also used to produce several semisynthetic derivatives, which together are known as the tetracycline antibiotics. The term tetracycline is also used to denote the 4-ring system of this compound; tetracyclines are related substances that contain the same 4-ring system.Contents
Mechanism of action
Tetracyclines bind to the 30S subunit of microbial ribosomes. They inhibit protein synthesis by blocking the attachment of charged aminoacyl-tRNA. Thus they prevent introduction of new amino acids to the nascent peptide chain.[1] The action is usually inhibitory and reversible upon withdrawal of the drug. Resistance to the tetracyclines results from changes in permeability of the microbial cell envelope. In susceptible cells, the drug is concentrated from the environment and does not readily leave the cell. In resistant cells, the drug is not actively transported into the cell or leaves it so rapidly that inhibitory concentrations are not maintained. This is often plasmid-controlled. Mammalian cells are not vulnerable to the effect of tetracyclines as these contain no 30S ribosomal subunit and therefore do not accumulate the drug.
History
The tetracyclines are a large family of antibiotics that were discovered as natural products by Benjamin Minge Duggar and first described in 1948.[2] Under Yellapragada Subbarao, Benjamin Duggar made his discovery of the first tetracycline antibiotic, Aureomycin, at Lederle Laboratories in 1945.[3]
In 1950, Harvard Professor Robert Woodward determined the chemical structure of the related substance, oxytetracycline (Terramycin); the patent[4] protection for its fermentation and production was also first issued in 1950. A research team of seven scientists (K.J. Brunings, Francis A. Hochstein, C.R. Stephens, L.H. Conover, Abraham Bavley, Richard Pasternack, and Peter P. Regna) at Pfizer,[5][6] in collaboration with Woodward, participated in the two-year research leading to the discovery.[7]
Pfizer was of the view that it deserved the right to a patent on tetracycline and filed its Conover application in October 1952. Cyanamid filed its Boothe Morton application for similar rights in March 1953 while Heyden Chemicals filed its Minieri application in September 1953, named after scientist P. Paul Minieri, in order to obtain a patent on tetracycline and its fermentation process. This resulted in Tetracycline litigation in which the winner would have to prove beyond reasonable doubt of priority invention and tetracycline’s natural state.[8]
Nubian mummies studied in the 1990s were found to contain significant levels of tetracycline; there is evidence that the beer brewed at the time could have been the source.[9] Tetracycline sparked the development of many chemically altered antibiotics, and in doing so has proved to be one of the most important discoveries made in the field of antibiotics.[citation needed] It is used to treat many Gram-positive and Gram-negative bacteria and some protozoa.[citation needed] Like some other antibiotics, it is also used in the treatment of acne.[citation needed]
Cautions, contraindications, side effects
Are as those of the tetracycline antibiotics group:[citation needed]
- Can stain developing teeth (even when taken by the mother during pregnancy)
- Can cause permanent teeth discoloration (yellow-gray-brown); infancy and childhood to eight years old
- Inactivated by Ca2+ ion, not to be taken with milk, yogurt, and other dairy products
- Inactivated by aluminium, iron and zinc, not to be taken at the same time as indigestion remedies
- Inactivated by common antacids and over-the-counter heartburn medicines
- Skin photo-sensitivity; exposure to the sun or intense light is not recommended
- Drug-induced lupus, and hepatitis
- Can induce microvesicular fatty liver.
- Tinnitus
- May interfere with methotrexate by displacing it from the various protein binding sites
- Can cause breathing complications as well as anaphylactic shock in some individuals
- Should be avoided during pregnancy as it may affect bone growth of the fetus
- Caution should be exercised in long term use with breastfeeding. Short-term use safe; bio-availability in milk is low to nil.[10]
In 2010, the FDA added tetracycline to its Adverse Event Reporting System (AERS).[11] The AERS contains a list of medications under investigation by the FDA for potential safety issues. The list is published quarterly and available online. The AERS cites a potential link between the use of tetracycline products and Stevens-Johnson syndrome, toxic epidermal necrolysis and erythema multiforme.[11]
Indications
It is first-line therapy for Rocky Mountain spotted fever (Rickettsia), Q fever (Coxiella), psittacosis and lymphogranuloma venereum (Chlamydia), and to eradicate nasal carriage of meningococci. Tetracycline tablets were used in the plague outbreak in India in 1992.[12]
Doxycycline is also one (of many) recommended drugs for chemoprophylatic treatment of malaria in travels to areas of the world where malaria is endemic.[13]
Other uses
Since tetracycline is absorbed into bone, it is used as a marker of bone growth for biopsies in humans. Tetracycline labeling is used to determine the amount of bone growth within a certain period of time, usually a period of approximately 21 days. Tetracycline is incorporated into mineralizing bone and can be detected by its fluorescence.[14] In double tetracycline labeling, a second dose is given 11–14 days after the first dose, and the amount of bone formed during that interval can be calculated by measuring the distance between the two fluorescent labels.[15]
Tetracycline is also used as a biomarker in wildlife to detect consumption of medicine- or vaccine-containing baits.[16]
In genetic engineering, tetracycline is used in transcriptional activation. Tetracycline is also one of the antibiotics used to treat ulcers caused by bacterial infections. In cancer research at Harvard Medical School, tetracycline has been used to switch off leukemia in genetically altered mice, and to do so reliably, when added to their drinking water.[17]
Cell culture
Tetracycline is used in cell biology as a selective agent in cell culture systems. It is toxic to prokaryotic and eukaryotic cells and selects for cells harboring the bacterial tetr gene, which encodes a 399-amino acid membrane-associated protein. This protein actively exports tetracycline from the cell, rendering cells harboring this gene more resistant to the drug. The yellow crystalline powder can be dissolved in water (20 mg/ml) or ethanol (5 mg/ml) and is routinely used at 10 mg/l in cell culture. In cell culture at 37 °C (99 °F) it is stable for days, with a half-life of approximately 24 hours.
Notes
- ^ Mechanism of Action of Tetracyclines
- ^ Klajn, Rafal, Chemistry and chemical biology of tetracyclines, retrieved 20 June 2007.
- ^ Jukes, Thomas H. Some historical notes on chlortetracycline. Reviews of Infectious Diseases 7(5):702-707 (1985).
- ^ U.S. Patent 2,516,080
- ^ "Coronagraph Mounts Done". The Science News 62 (6): 83. 1952. doi:10.2307/3931295. JSTOR 3931295.
- ^ "Scientists Discover Terramycin's Secret: Its Complex Structure". http://pqasb.pqarchiver.com/djreprints/access/107482962.html?dids=107482962:107482962&FMT=ABS&FMTS=ABS:AI&type=historic&date=Jul+28%2C+1952&author=&pub=Wall+Street+Journal&desc=Scientists+Discover+Terramycin's+Secret%3A+Its+Complex+Structure&pqatl=google.
- ^ {{Cite journal Prior to 1952, neither the molecular structure of Terramycin nor that of Aureomycin was known. In the spring of 1952 the Pfizer team succeeded in ascertaining the structures of both Terramycin and Aureomycin. Shortly thereafter, L.H. Conover discovered that another antibiotic, tetracycline, could be produced by the deschlorination of Aureomycin. Pfizer filed the application for a product and process patent on tetracycline in October of 1952, and in March of 1953 Cyanamid filed its Boothe-Morton application for a similar patent. In addition to these two applications, in September 1953 Heyden Chemical Corporation filed for a patent on tetracycline and the fermentation process for producing it in the name of P. Paul Minieri, and in October 1953, Bristol filed a similar application under the name of Heinemann. Because of an agreement among the major drug companies to cross-license tetracyline, Fair Trade Practices litigation was initiated which was not resolved until 982. The Federal Trade Commission argued that Pfizer, American Cyanamid (successor to Heyden), Bristol-Myers and others had conspired to fix prices for the new antibiotic. The FTC had argued that because tetracycline was produced through fermentation, rather than synthetically, that it was not patentable, and its distribution was subject to pricing fixing challenge. The FTC also argued that tetracycline was not patentable because of its production through fermentation. | last1 = Hochstein | first1 = F. A. | last2 = Stephens | first2 = C. R. | last3 = Conover | first3 = L. H. | last4 = Regna | first4 = P. P. | last5 = Pasternack | first5 = R. | last6 = Gordon | first6 = P. N. | last7 = Pilgrim | first7 = F. J. | last8 = Brunings | first8 = K. J. | last9 = Woodward | first9 = R. B. | title = The structure of terramycin | journal = Journal of the American Chemical Society | volume = 75 | issue = 22 | pages = 5455–75 | year = 1953 | month = November | doi = 10.1021/ja01118a001 }}
- ^ Patented Feb 7, 1956 http://commons.wikimedia.org/wiki/File:Tetracycline.pdf
- ^ George Armelagos (May 2000). "Take Two Beers and Call Me in 1,600 Years - use of tetracycline by Nubians and Ancient Egyptians". American Museum of Natural History. http://www.findarticles.com/p/articles/mi_m1134/is_4_109/ai_62324477. Retrieved 2007-12-19.
- ^ Riordan,Jan."Breastfeeding & Human Lactation",Jones & Bartlett,2010 p.179
- ^ a b FDA Adverse Events Reporting System Retrieved on January 14, 2011
- ^ Lippincott's Illustrated Reviews: Pharmacology, 4th ed. Harvery RA, Champe, PC. Lippincott, Williams & Wilkins, 2009
- ^ Chapter 2 - Malaria - 2010 Yellow Book | CDC Travelers' Health
- ^ Mayton CA. Tetracycline labeling of bone
- ^ The Johns Hopkins Medical Institutions. > Tetracycline Labeling Last updated January 8, 2001.
- ^ Olson CA, Mitchell KD, Werner PA (October 2000). "Bait ingestion by free-ranging raccoons and nontarget species in an oral rabies vaccine field trial in Florida". J. Wildl. Dis. 36 (4): 734–43. PMID 11085436. http://www.jwildlifedis.org/cgi/reprint/36/4/734.
- ^ William J. Cromie (February 10, 2000). "Researchers Switch Cancer Off and On -- In Mice". Harvard Gazette. http://news.harvard.edu/gazette/2000/02.10/leukemia.html. Retrieved 2008-10-25.
Stomatological preparations (A01) Caries prophylactic agents Anti-infectives and antiseptics Amphotericin B • Benzoxonium chloride • Chlorhexidine • Chlortetracycline • Clotrimazole • Domiphen bromide • Doxycycline • Eugenol • Hexetidine • Hydrogen peroxide • Mepartricin • Metronidazole • Miconazole • Minocycline • Natamycin • Neomycin • Oxyquinoline • Polynoxylin • Sodium perborate • Tetracycline • Tibezonium iodideCorticosteroids (Glucocorticoids) Other Antibiotics and chemotherapeutics for dermatological use (D06) Antibiotics Tetracycline and derivativesOthersChemotherapeutics Aciclovir • Penciclovir • Idoxuridine • Edoxudine
Imiquimod/Resiquimod • Podophyllotoxin
Docosanol • Tromantadine • Inosine • Lysozyme • IbacitabineOtherAcne-treating agents (D10) Antibacterial Keratolytic Anti-inflammatory Antibiotics Hormonal Retinoids Combinations Adapalene/benzoyl peroxide • Benzoyl peroxide/clindamycin • Clindamycin/tretinoin • Erythromycin/isotretinoin • Sulfacetamide/sulfurAntibacterials: protein synthesis inhibitors (J01A, J01B, J01F, J01G, QJ01XQ) 30S -mycin (Streptomyces)Neomycin# (Framycetin, Paromomycin, Ribostamycin)
Kanamycin# (Amikacin, Arbekacin, Bekanamycin, Dibekacin, Tobramycin)
Paromomycin-micin (Micromonospora)TetracyclinesDoxycycline# •Chlortetracycline • Clomocycline • Demeclocycline • Lymecycline • Meclocycline • Metacycline • Minocycline • Oxytetracycline • Penimepicycline • Rolitetracycline • Tetracycline50S Linezolid • Torezolid • Eperezolid • Posizolid • RadezolidPleuromutilinsRetapamulin • Tiamulin • ValnemulinErythromycin# • Azithromycin# • Spiramycin • Midecamycin • Oleandomycin • Roxithromycin • Josamycin • Troleandomycin • Clarithromycin • Miocamycin • Rokitamycin • Dirithromycin • Flurithromycin • Ketolide (Telithromycin, Cethromycin, Solithromycin)EF-G Steroid antibacterialsOtologicals (S02) Anti-infectives Acetic acid • Aluminium acetotartrate • Boric acid • Chloramphenicol • Chlorhexidine • Ciprofloxacin • Clioquinol • Gentamicin • Hydrogen peroxide • Miconazole • Neomycin • Nitrofurazone • Ofloxacin • Polymyxin B • Rifamycin • TetracyclineCorticosteroids Analgesics and anesthetics M: EAR
anat(e/p)/phys/devp
noco/cong, epon
proc, drug(S2)
Categories:- Amides
- Anti-acne preparations
- Cancer research
- Otologicals
- Tetracycline antibiotics
Wikimedia Foundation. 2010.
