- Cyanuric chloride
-
Cyanuric chloride 
 2,4,6-Trichloro-1,3,5-triazineOther namesTrichlorotriazine
2,4,6-Trichloro-1,3,5-triazineOther namesTrichlorotriazine
s-Triazine trichloride
Cyanuryl chlorideIdentifiers CAS number 108-77-0 
PubChem 7954 ChemSpider 7666 
EC number 203-614-9 UN number 2670 ChEBI CHEBI:58964 
RTECS number XZ1400000 Jmol-3D images Image 1 - Clc1nc(Cl)nc(Cl)n1
Properties Molecular formula C3Cl3N3 Molar mass 184.41 g/mol Appearance White powder Density 1.32 g/cm3 Melting point 154 °C
Boiling point 192 °C
Solubility in water hydrolyzes Solubility in organic solvents soluble Hazards MSDS ICSC 1231 EU Index 613-009-00-5 EU classification Very toxic (T+)
Harmful (Xn)
Corrosive (C)R-phrases R14, R22, R26, R34, R43 S-phrases (S1/2), S26, S28, S36/37/39, S45, S46, S63 NFPA 704 Flash point Non-flammable Related compounds Related triazines Cyanuric acid
Cyanuric fluoride
Trichloroisocyanuric acid chloride (verify) (what is:
chloride (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Cyanuric chloride is the inorganic compound with the formula (NCCl)3. This colorless solid is the chlorinated derivative of 1,3,5-triazine. It is the trimer of cyanogen chloride.[1] Cyanuric chloride is the main precursor to the popular but controversial herbicide atrazine.
Contents
Production
Cyanuric chloride is prepared in two steps from hydrogen cyanide via the intermediacy of cyanogen chloride, which is trimerized at elevated temperatures over a carbon catalyst:
- HCN + Cl2 → ClCN + HCl
- 3 ClCN → (ClCN)3
In 2005, approximately 200,000 tonnes were produced.[2]
Industrial Uses
It is estimated that 70% of cyanuric chloride is used in the preparation of the triazine-class pesticides, especially atrazine. Such reactions rely on the easy displacement of the chloride with nucleophiles such as amines:
- (ClCN)3 + 2 RNH2 → (RNHCN)(ClCN)2 + RNH3+Cl-
Other triazine herbicides, such as simazine, anilazine and cyromazine are made in an analogous way.[3]
Cyanuric chloride is also used as a precursor to dyes and crosslinking agents. The largest class of these dyes are the sulfonated triazine-stilbene optical brighteners (OBA) or fluorescent whitening agents (FWA) commonly found in detergent formulas and white paper.[2] Many reactive dyes also incorporate a triazine ring. They are also manufactured by way of the chloride displacement reaction shown above.[3]
Organic synthesis
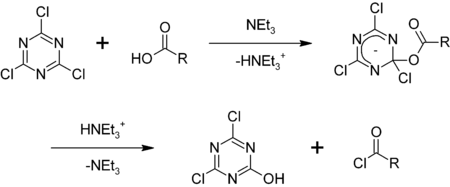
In one specialized application, cyanuric chloride is employed as a reagent in organic synthesis for the conversion of alcohols and carboxylic acids into alkyl and acyl chlorides, respectively:[4]
It is also used as a dehydrating agent and for the activation of carboxylic acids for reduction to alcohols. Heating with DMF gives "Gold's reagent" Me2NCH=NCH=NMe2+Cl-, which is a versatile source of aminoalkylations and a precursor to heterocycles.[5][6]
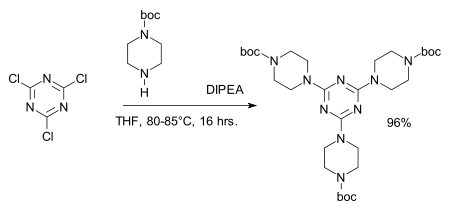
The chloride centers are easily replaced by amines to give melamine derivatives, for example in the synthesis of dendrimers:[7][8]
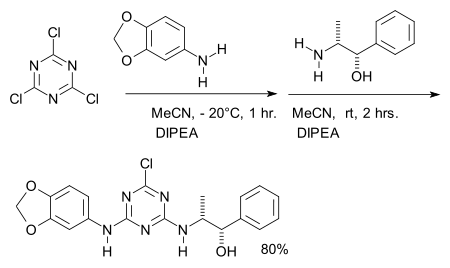
It is also employed the synthesis of an experimental adenosine receptor ligand.[9]:
Cyanuric Chloride can also be used as an alternative to oxalyl chloride in the Swern oxidation[10].
References
- ^ Cyanuric chloride at Chemicalland21.com
- ^ a b Klaus Huthmacher, Dieter Most "Cyanuric Acid and Cyanuric Chloride" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a08_191.
- ^ a b Ashford's Dictionary of Industrial Chemicals, 3rd edition, 2011, pages 2495-8
- ^ K. Venkataraman, and D. R. Wagle (1979). "Cyanuric chloride : a useful reagent for converting carboxylic acids into chlorides, esters, amides and peptides". Tet. Lett. 20 (32): 3037–3040. doi:10.1016/S0040-4039(00)71006-9.
- ^ Probst, D. A.; Hanson, P. R.; Barda, D. A. "Cyanuric Chloride" in Encyclopedia of Reagents for Organic Synthesis, 2004, John Wiley & Sons. doi:10.1002/047084289X.rn00320
- ^ John T. Gupton; Steven A. Andrews (1990), "β-Dimethylaminomethylenation: N,N-Dimethyl-N'-p-tolylformamidine", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv7p0197; Coll. Vol. 7: 197
- ^ Abdellatif Chouai and Eric E. Simanek (2008). "Kilogram-Scale Synthesis of a Second-Generation Dendrimer Based on 1,3,5-Triazine Using Green and Industrially Compatible Methods with a Single Chromatographic Step". J. Org. Chem. 73 (6): 2357–2366. doi:10.1021/jo702462t. PMID 18307354.
- ^ Reagent: DIPEA, amine protective group: BOC
- ^ WO application 03101980, "1,3,5-TRIAZINE DERIVATIVES AS LIGANDS FOR HUMAN ADENOSINE-A3 RECEPTORS", published 2003-12-11 (Reagent number two: norephedrine, base DIPEA)
- ^ http://pubs.acs.org/doi/abs/10.1021/jo015935s De Luca, L.; Giacomelli, G.; Procheddu, A. J. Org. Chem. 2001, 7907.
Categories:- Inorganic chlorine compounds
- Triazines
- Dehydrating agents
Wikimedia Foundation. 2010.