- Nitrilase
-
nitrilase 1 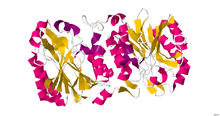
Crystallographic structure of Pyrococcus abyssi nitrilase.[1] Identifiers Symbol NIT1 Entrez 4817 HUGO 7828 OMIM 604618 PDB 3IVZ RefSeq NM_005600 UniProt Q86X76 Other data Locus Chr. 1 pter-qter Nitrilase enzymes (nitrile aminohydrolase; EC 3.5.5.1) catalyse the hydrolysis of nitriles to carboxylic acids and ammonia, without the formation of "free" amide intermediates. Nitrilases are involved in natural product biosynthesis and post translational modifications in plants, animals, fungi and certain prokaryotes. Nitrilases can also be used as catalysts in preparative organic chemistry. Among others, nitrilases have been used for the resolution of racemic mixtures. Nitrilase should not be confused with nitrile hydratase (nitrile hydro-lyase; EC 4.2.1.84) which hydrolyses nitriles to amides. Nitrile hydratases are almost invariably co-expressed with an amidase, which converts the amide to the carboxylic acid, consequently it can sometimes be difficult to distinguish nitrilase activity from nitrile hydratase plus amidase activity.
Nitrilase is a polypeptide consisting of alpha helices and beta sheets. It is 262 residues in length and its structure was determined through [x-ray diffraction]. The structure of nitrilase shown has a resolution of 1.57 [angstroms] (Å) or 0.157 nanometers (nm). Nitrilase is an enzyme and therefore requires a [ligand] in order for it to become active. Magnesium ion is the ligand that is a tightly bound to the enzyme to allow for this activation. This information was taken from Protein Data Bank
References
External links
Categories:- Genes on chromosome 1
- Enzyme stubs
Wikimedia Foundation. 2010.
