- Hexane
-
n-Hexane 


 HexaneOther namesn-Hexane
HexaneOther namesn-HexaneIdentifiers CAS number 110-54-3 
PubChem 8058 ChemSpider 7767 
UNII 2DDG612ED8 
DrugBank DB02764 KEGG C11271 
ChEBI CHEBI:29021 
ChEMBL CHEMBL15939 
RTECS number MN9275000 Jmol-3D images Image 1 - CCCCCC
Properties Molecular formula C6H14 Molar mass 86.18 g mol−1 Appearance Colorless liquid Density 0.6548 g/mL Melting point −95 °C, 178 K, -139 °F
Boiling point 69 °C, 342 K, 156 °F
Solubility in water 13 mg/L at 20°C[1] Viscosity 0.294 cP Hazards MSDS External MSDS EU classification Flammable (F)
Harmful (Xn)
Repr. Cat. 3
Dangerous for
the environment (N)R-phrases R11 R38 R48/20 R62 R65 R67 R51/53 S-phrases (S2) S9 S16 S29 S33 S36/37 S61 S62 NFPA 704 Flash point −23.3 °C Autoignition
temperature233.9 °C Related compounds Related alkanes Pentane
HeptaneRelated compounds Cyclohexane Supplementary data page Structure and
propertiesn, εr, etc. Thermodynamic
dataPhase behaviour
Solid, liquid, gasSpectral data UV, IR, NMR, MS  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Hexane is a hydrocarbon with the chemical formula C6H14; that is, an alkane with six carbon atoms.
The term may refer to any of four other structural isomers with that formula, or to a mixture of them.[2] In the IUPAC nomenclature, however, hexane is the unbranched isomer (n-hexane); the other four structures are named as methylated derivatives of pentane and butane. IUPAC also uses the term as the root of many compounds with a linear six-carbon backbone, such as 2-methylhexane C7H16, which is also called "isoheptane".
Hexanes are significant constituents of gasoline. They are all colorless liquids at room temperature, with boiling points between 50 and 70 °C, with gasoline-like odor. They are widely used as cheap, relatively safe, largely unreactive, and easily evaporated non-polar solvents.
Contents
Isomers
Common name IUPAC name Text formula Skeletal formula normal hexane
n-hexanehexane CH3(CH2)4CH3 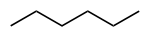
isohexane 2-methylpentane (CH3)2CH(CH2)2CH3 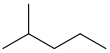
3-methylpentane CH3CH2CH(CH3)CH2CH3 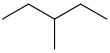
2,3-dimethylbutane CH3CH(CH3)CH(CH3)CH3 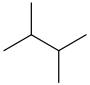
neohexane 2,2-dimethylbutane CH3C(CH3)2CH2CH3 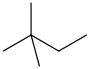
Uses
In industry, hexanes are used in the formulation of glues for shoes, leather products, and roofing. They are also used to extract cooking oils from seeds, for cleansing and degreasing all sorts of items, and in textile manufacturing.
A typical laboratory use of hexanes is to extract oil and grease contaminants from water and soil for analysis.[3] Since hexane cannot be easily deprotonated, it is used in the laboratory for reactions that involve very strong bases, such as the preparation of organolithiums, e.g. Butyllithiums are typically supplied as a hexane solution.
In many applications (especially pharmaceutical), the use of n-hexane is being phased out due to its long term toxicity, and often replaced by n-heptane, which will not form the toxic (hexane-2,5-dione) metabolite.
Production
Hexanes are chiefly obtained by the refining of crude oil. The exact composition of the fraction depends largely on the source of the oil (crude or reformed) and the constraints of the refining. The industrial product (usually around 50% by weight of the straight-chain isomer) is the fraction boiling at 65–70 °C.
Physical properties
The boiling points of the various hexanes are somewhat similar and, as for other alkanes, are generally lower for the more branched forms. The melting points are quite different and the trend is not apparent.[4]
Isomer M.P. (°C) B.P. (°C) n-hexane −95.3 68.7 3-methylpentane −118.0 63.3 2-methylpentane (isohexane) −153.7 60.3 2,3-dimethylbutane −128.6 58.0 2,2-dimethylbutane (neohexane) −99.8 49.7 Normal hexane has considerable vapor pressure at room temperature:[5]
temperature (°C) vapor pressure (mmHg) −40 3.36 −30 7.12 −20 14.01 −10 25.91 0 45.37 10 75.74 20 121.26 25 151.28 30 187.11 40 279.42 50 405.31 60 572.76 Toxicity
The acute toxicity of hexane is relatively low, although it is a mild anesthetic. Inhalation of high concentrations produces first a state of mild euphoria, followed by somnolence with headaches and nausea.
The long-term toxicity of n-hexane in humans is well known.[6] Extensive peripheral nervous system failure is known to occur in humans chronically exposed to levels of n-hexane ranging from 400 to 600 ppm, with occasional exposures up to 2,500 ppm. The initial symptoms are tingling and cramps in the arms and legs, followed by general muscular weakness. In severe cases, atrophy of the skeletal muscles is observed, along with a loss of coordination and problems of vision. Similar symptoms are observed in animal models. They are associated with a degeneration of the peripheral nervous system (and eventually the central nervous system), starting with the distal portions of the longer and wider nerve axons. The toxicity is not due to hexane itself but to one of its metabolites, hexane-2,5-dione. It is believed that this reacts with the amino group of the side chain of lysine residues in proteins, causing cross-linking and a loss of protein function.
Chronic intoxication from hexane has been observed in recreational solvent abusers and in workers in the shoe manufacturing, furniture restoration and automobile construction industries, and recently, plastic recyclers and assemblers and cleaners of capacitive touch-screen devices.[7]
In 1994, n-hexane was included in the list of chemicals on the US Toxic Release Inventory (TRI).[8] In 2001, the U.S. Environmental Protection Agency issued regulations on the control of emissions of hexane gas due to its potential carcinogenic properties and environmental concerns.[9]
Use in food processing
According to a report by the Cornucopia Institute, hexane is used to extract oil from grains as well as protein from soy, to such an extent that in 2007, grain processors were responsible for more than two-thirds of hexane emissions in the United States.[10] The report also pointed out that the hexane can persist in the final food product created; in a sample of processed soy, the oil contained 10 ppm, the meal 21 ppm and the grits 14 ppm hexane.[10] The adverse health effects seem specific to n-hexane; they are much reduced or absent for other isomers. Therefore, the food oil extraction industry, which relied heavily on hexane, has been considering switching to other solvents, including isohexane.[11][12][13]
Poisoning from touchscreen cleaner
In February 2010, reports surfaced saying that an employee of Wintek Corporation in China, a company that manufactures touchscreen components, died in August 2009 due to hexane poisoning. Hexane was used as a replacement for alcohol for cleaning the screens. Reports suggest up to 137 Chinese employees required treatment for hexane poisoning around the same time.[14][15][16] An ABC Foreign Correspondent episode covertly interviewed several women who had been in the hospital for over six months. The women claimed that they were exposed to hexane while manufacturing iPhone hardware.[17][18]
See also
References
- ^ n-Hexane, Date of Peer Review: April 2000
- ^ "C5 and C6 alkanes". A and B Scott Organic Chemistry. http://members.optushome.com.au/scottsoftb/skeletons2.htm. Retrieved 2007-10-30.
- ^ Use of ozone depleting substances in laboratories. TemaNord 2003:516.
- ^ William D. McCain (1990), The properties of petroleum fluids. PennWell. ISBN 0-87814-335-1
- ^ vapor pressure
- ^ Hathaway GJ, Proctor NH, Hughes JP, and Fischman M (1991). Proctor and Hughes' chemical hazards of the workplace. 3rd ed. New York, NY: Van Nostrand Reinhold.
- ^ Dirty Secrets ABC News Broadcast: 26/10/2010
- ^ "N-Hexane Chemical Backgrounder". National Safety Council. Archived from the original on 19 May 2007. http://web.archive.org/web/20070519002303/http://www.nsc.org/ehc/chemical/N-Hexane.htm. Retrieved 25 May 2007.
- ^ Anuradee Witthayapanyanon and Linh Do. "Nanostructured Microemulsions as Alternative Solvents to VOCs in Cleaning Technologies and Vegetable Oil Extraction". National Center For Environmental Research. Archived from the original on 13 October 2007. http://web.archive.org/web/20071013110614/http://es.epa.gov/ncer/publications/meetings/10_26_05/abstracts/do.html. Retrieved 25 May 2007.
- ^ a b Cornucopia Institute (2009-05-18) Behind the Bean - The Heroes and Charlatans of the Natural and Organic Soy Foods Industry
- ^ Peter J. Wan, Phillip J. Wakelyn (1997), Technology and solvents for extracting oilseeds and nonpetroleum oils The American Oil Chemists Society. ISBN 0-935315-81-0, p. 189
- ^ USDA (1996), Isohexane—New Solvent for Cottonseed Oil Processing
- ^ Ono, Yuichiro; Takeuchi, Yasuhiro; Hisanaga, Naomi (1981). "A comparative study on the toxicity of n-hexane and its isomers on the peripheral nerve". International Archives of Occupational and Environmental Health 48 (3): 289–294. doi:10.1007/BF00405616. PMID 7251182.
- ^ Worker Dies From N-hexane Poisoning At Touchscreen Factory Gizmodo, February 23, 2010
- ^ Silicon Sweatshops: Another black eye for Apple supplier Global Post, February 25, 2010
- ^ iPhone workers still sick after chemical poisoning IDG News Service February, 22 2011
- ^ Workers poisoned while making iPhones ABC News, October 25, 2010
- ^ Dirty Secrets ABC Foreign Correspondent, 2010-Oct-26
External links
- International Chemical Safety Card 1262 (2-methylpentane)
- Material Safety Data Sheet for Hexane
- National Pollutant Inventory - n-hexane fact sheet
- NIOSH Pocket Guide to Chemical Hazards (hexane isomers)
- Phytochemica l database entry
- Center for Disease Control and Prevention
- Warning from National Safety Council "COMMON CHEMICAL AFFECTS AUTO MECHANICS"
- Australian National Pollutant Inventory (NPI) page
- "EPA does not consider n-hexane classifiable as a human carcinogen." Federal Register / Vol. 66, No. 71 / Thursday, April 12, 2001 / Rules and Regulations
Alkanes Higher alkanes · List of alkanesCategories:- Alkanes
- Hazardous air pollutants
- Hydrocarbon solvents
Wikimedia Foundation. 2010.

