- Drude model
-
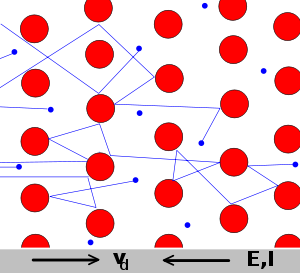
The Drude model of electrical conduction was proposed in 1900[1][2] by Paul Drude to explain the transport properties of electrons in materials (especially metals). The model, which is an application of kinetic theory, assumes that the microscopic behavior of electrons in a solid may be treated classically and looks much like a pinball machine, with a sea of constantly jittering electrons bouncing and re-bouncing off heavier, relatively immobile positive ions.
The two most significant results of the Drude model are an electronic equation of motion,
and a linear relationship between current density J and electric field E,
Here t is the time and p, q, n and m, and τ are respectively an electron's momentum, charge, number density, mass, and mean free time between ionic collisions. The latter expression is particularly important because it explains in semi-quantitative terms why Ohm's Law, one of the most ubiquitous relationships in all of electromagnetism, should be true.[3][4][5]
The model was extended in 1905 by Hendrik Antoon Lorentz (and hence is also known as the Drude–Lorentz model) and was supplemented with the results of quantum theory in 1933 by Arnold Sommerfeld and Hans Bethe.
Contents
Assumptions
The Drude model considers the metal to be formed of a mass of positively-charged ions from which a number of "free electrons" were detached. These may be thought to have become delocalized when the valence levels of the atom came in contact with the potential of the other atoms.[6]
Notwithstanding the origin of such free electrons from the contact of different potentials, the Drude model neglects any long-range interaction between the electron and the ions and assumes that the electrons do not interfere with each other. The only possible interaction is the instantaneous collision between a free electron and an ion, which happens with a fixed probability per unit time.[6]
The Drude model is a purely classical model, and treats both electrons and ions as solid spheres.
Explanations
DC field
The simplest analysis of the Drude model assumes that electric field
 is both uniform and constant, and that the thermal velocity of electrons is sufficiently high such that they accumulate only an infinitesimal amount of momentum
is both uniform and constant, and that the thermal velocity of electrons is sufficiently high such that they accumulate only an infinitesimal amount of momentum  between collisions, which occur on average every τ seconds.[3]
between collisions, which occur on average every τ seconds.[3]Then an electron isolated at time t will on average have been traveling for time τ since its last collision, and consequently will have accumulated momentum
During its last collision, this electron will have been just as likely to have bounced forward as backward, so all prior contributions to the electron's momentum may be ignored, resulting in the expression
Substituting the relations
results in the formulation of Ohm's Law mentioned above:
Time-varying analysis
The dynamics may also be described by introducing an effective drag force. At time t = t0 + dt the average electron's momentum will be
because, on average, (1 − dt / τ) electrons will not have experienced another collision, and the ones that have will contribute to the total momentum to only a negligible order.[7]
With a bit of algebra, this results in the differential equation
where
 denotes average momentum, m the effective mass and q the charge of the electrons. This, which is an inhomogeneous differential equation, may be solved to obtain the general solution of
denotes average momentum, m the effective mass and q the charge of the electrons. This, which is an inhomogeneous differential equation, may be solved to obtain the general solution offor p(t). The steady state solution (
 ) is then
) is thenAs above, average momentum may be related to average velocity and this in turn may be related to current density,
and the material can be shown to satisfy Ohm's Law with a DC-conductivity
 :
:The Drude model can also predict the current as a response to a time-dependent electric field with an angular frequency
 , in which case
, in which caseHere it is assumed that
In other conventions,
 is replaced by
is replaced by  in all equations. The imaginary part indicates that the current lags behind the electrical field, which happens because the electrons need roughly a time
in all equations. The imaginary part indicates that the current lags behind the electrical field, which happens because the electrons need roughly a time  to accelerate in response to a change in the electrical field. Here the Drude model is applied to electrons; it can be applied both to electrons and holes; i.e., positive charge carriers in semiconductors. The curves for σ(ω) are shown in the graph.
to accelerate in response to a change in the electrical field. Here the Drude model is applied to electrons; it can be applied both to electrons and holes; i.e., positive charge carriers in semiconductors. The curves for σ(ω) are shown in the graph.Accuracy of the model
This simple classical Drude model provides a very good explanation of DC and AC conductivity in metals, the Hall effect, and thermal conductivity (due to electrons) in metals near room temperature. The model also explains the Wiedemann-Franz law of 1853. However, it greatly overestimates the electronic heat capacities of metals. In reality, metals and insulators have roughly the same heat capacity at room temperature. Although the model can be applied to positive (hole) charge carriers, as demonstrated by the Hall effect, it does not predict their existence.
One note of trivia surrounding the theory is that in his original paper Drude made a conceptual error, estimating electrical conductivity to in fact be only half of what it classically should have been.[8]
See also
References
- ^ Drude, Paul (1900). "Zur Elektronentheorie der metalle". Annalen der Physik 306 (3): 566. Bibcode 1900AnP...306..566D. doi:10.1002/andp.19003060312. http://www3.interscience.wiley.com/cgi-bin/fulltext/112485959/PDFSTART.
- ^ Drude, Paul (1900). "Zur Elektronentheorie der Metalle; II. Teil. Galvanomagnetische und thermomagnetische Effecte". Annalen der Physik 308 (11): 369. Bibcode 1900AnP...308..369D. doi:10.1002/andp.19003081102. http://www3.interscience.wiley.com/cgi-bin/fulltext/112485893/PDFSTART.
- ^ a b Neil W. Ashcroft; N. David Mermin (1976). Solid State Physics. Saunders College. pp. 6–7. ISBN 0-030839-939.
- ^ Edward M. Purcell (1965). Electricity and Magnetism. McGraw-Hill. pp. 117–122. ISBN 978-0-070049-086.
- ^ David J. Griffiths (1999). Introduction to Electrodynamics. Prentice-Hall. pp. 289. ISBN 978-0138053260.
- ^ a b Neil W. Ashcroft; N. David Mermin (1976). Solid State Physics. Saunders College. pp. 2–3. ISBN 0-030-83993-9.
- ^ Neil W. Ashcroft; N. David Mermin (1976). Solid State Physics. Saunders College. pp. 11. ISBN 0-030-83993-9.
- ^ Neil W. Ashcroft; N. David Mermin (1976). Solid State Physics. Saunders College. pp. 23. ISBN 0-03-083993-9.
Atomic Models Single atoms Dalton model (Billiard Ball Model) · Thomson model (Plum Pudding Model) · Lewis model (Cubical Atom Model) · Nagaoka model (Saturnian Model) · Rutherford model (Planetary Model) · Bohr model (Rutherford–Bohr Model) · Bohr–Sommerfeld model (Refined Bohr Model) · Gryziński model (Free-fall Model) · Schrodinger model (Electron Cloud Model)Atoms in solids Drude model · Free electron model · Nearly-free electron model · Band structure · Density functional theoryAtoms in liquids Atoms in gases Scientists Categories:
Wikimedia Foundation. 2010.