- Methylenedioxypyrovalerone
-
Methylenedioxypyrovalerone 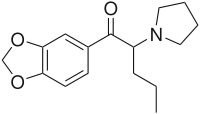

Systematic (IUPAC) name (RS)-1-(Benzo[d][1,3]dioxol-5-yl)-2-(pyrrolidin-1-yl)pentan-1-one Clinical data Pregnancy cat. ? Legal status Unscheduled (illegal in Czech Republic, Denmark and Sweden) Routes Oral, Insufflation, Intravenous, Rectal, Vaporization Pharmacokinetic data Metabolism Hepatic Excretion Primarily Urine (Renal) Identifiers CAS number 687603-66-3 
24622-62-6 (HCl)ATC code ? PubChem CID 20111961 ChemSpider 16788110 
Chemical data Formula C16H21NO3 Mol. mass 275.343 g/mol (freebase) Physical data Melt. point 209.3 °C (409 °F) Boiling point 476 °C (889 °F)  (what is this?) (verify)
(what is this?) (verify)Methylenedioxypyrovalerone (MDPV) is a psychoactive drug with stimulant properties which acts as a norepinephrine-dopamine reuptake inhibitor (NDRI). Reportedly, it has been sold since around 2004 as a designer drug. It is also known as MDPK, MTV, Magic, Maddie, Black Rob, Super Coke and PV.[1] In 2010 it was reportedly sold as a legal drug alternative.[2][3]
Incidences of psychological and physical harm have been attributed to MDPV use.[4][5]
Contents
Appearance
The hydrochloride salt exists as a very fine, hygroscopic, crystalline powder that tends to clump to itself, resembling something like powdered sugar. Its color can range from pure white to a yellowish-tan and has a slight odor that strengthens as it colors. Impurities are likely to consist of either pyrollidine or alpha-dibrominated alkylphenones from either excess pyrollidine or incomplete amination, respectively, during synthesis and likely accounts for its discoloration and fishy (pyrollidine) or bromine-like odor, which worsens upon exposure to air, moisture, or bases.[6][7]
Pharmacology
MDPV has no history of FDA approved medical use.[8] Reportedly, it has four times the potency of methylphenidate (Ritalin, Concerta).[9] MDPV is the 3,4-methylenedioxy ring-substituted analog of the compound pyrovalerone, developed in the 1960s, which has been used for the treatment of chronic fatigue and as an anorectic, but caused problems of abuse and dependence.[1] However, despite its structural similarity, the effects of MDPV bear little resemblance to other methylenedioxyphenylalkylamine derivatives such as 3,4-methylenedioxy-N-methylamphetamine (MDMA), instead producing primarily stimulant effects with only mild entactogenic qualities.[1]
Other drugs with a similar chemical structure include α-pyrrolidinopropiophenone (α-PPP), 4'-methyl-α-pyrrolidinopropiophenone (MPPP), 3',4'-methylenedioxy-α-pyrrolidinopropiophenone (MDPPP) and 1-phenyl-2-(1-pyrrolidinyl)-1-pentanone (α-PVP).
Effects
MDPV acts as a stimulant and has been reported to produce effects similar to those of cocaine, methylphenidate, and amphetamines.[1] The acute effects may include:[1][10]
Physiological/Psychological effects
- tachycardia (Rapid heartbeat)
- hypertension (High blood pressure)
- vasoconstriction (Narrowing of the blood vessels)
- insomnia (Inability to sleep)
- nausea, stomach cramps, and digestive problems
- bruxism (Grinding teeth)
- increased body temperature, chills, sweating
- pupil dilation
- headache
- kidney pain
- tinnitus
- dizziness
- overstimulation
- breathing difficulty
- agitation/hypertonia
- severe paranoia
- confusion
- psychotic delusions
- extreme anxiety/agitation, sometimes progressing to violent behavior
- suicidal thoughts/actions
Psychiatric symptoms may persist. Physical symptoms may progress to rhabdomyolysis, renal failure, seizures, high anion gap metabolic acidosis, respiratory failure, or liver failure.
Desired psychological effects
- euphoria
- increased alertness and awareness
- increased wakefulness and arousal
- increased energy and motivation
- mental stimulation/increased concentration
- increased sociability
- sexual stimulation/aphrodisiac effects
- mild empathogenic effects
- diminished perception of the requirement for food and sleep
- modification of the symptom profile in early stages of opiate withdrawal consistent with its dopamine reputake inhibitor function; reportedly, attempting to use the drug in management of withdrawal is impossible because of a spectacular (in a neutral or bad sense) and unmanageable complex of side effects, as described in this article, rapidly supervening. The tendency to inhibit norepinephrine reputake would cause a progressive relative worsening of some physical symptoms of opiate withdrawal, generally those that would be subtracted out by clonidine therapy.[11]
Description of effects
The primary psychological effects have a duration of roughly 3 to 4 hours, with after effects such as tachycardia, hypertension, and mild stimulation lasting from 6 to 8 hours.[1][10] High doses have been observed to cause intense, prolonged panic attacks in stimulant-intolerant users,[1][10] and there are anecdotal reports of psychosis from sleep withdrawal and addiction at higher doses or more frequent dosing intervals.[1][10] MDPV has been distinguished by some for its powers as an aphrodisiac.[1] It has also been repeatedly noted for inducing strong cravings to re-administer.[10][12] Users have reported a compulsive desire to continuously re-dose, even following onset of the unpleasant side effects induced by prolonged use and higher doses.
Extended binges on MDPV have also been reported to produce severe comedown syndrome similar to that of methamphetamine[citation needed], characterized by depression, lethargy, headache, anxiety, postural hypotension (lightheadedness and weakness of the muscles), and in some cases severely bloodshot eyes, which usually subside within 4 to 8 hours. MDPV may also cause temporary bruxism. Side effects are highly dose-dependent. No fatalities have so far been reported without the combination of other substances except for suicide.[1]
Reported modalities of intake include oral consumption, insufflation, smoking, rectal and intravenous use. It is supposedly active at 3–5 mg, with typical doses ranging between 5–20 mg.[1] MDPV loses potency when it is put into solution.[10]
Chemistry
MDPV can be prepared by modifying the alkylation-oxidation-bromination-amination route to cathinone analogs. This involves a Grignard alkylation of piperonal, oxidation of the resulting secondary alcohol back into a ketone, alpha halogenation of the aromatic ketone with bromine, and subsequent amination with pyrrolidine.[13]
The α,α-dibrominated or α-mono-brominated intermediate from the 3rd step bromination, and left behind by an expedited or incomplete final workup is the most likely contaminant/impurity to be seen in the final product using this method.[6] Bromination is likely to in excess, since excess pyrollidine amine will form a black precipitate that is difficult to separate.
Such brominated halo are of particular concern to eukaryote macro-biological organisms, and special consideration must be made when preparing MDPV for purposes of long-term biological testing on lab animals, especially in mammals, and especially where the possibility exists for diversion and subsequent ingestion by humans for recreational purposes. Preparation for biological purposes and testing must ensure a complete and through workup, including several washes with saturated NaHCO3[6] solution after washing with water to remove the brominated intermediates before final salting of the freebase for recrystallization. Aside from the bromine ion being highly electronegative and reactive, the alkyl-bromine compounds often being alkylating agents, and brominated aromatic derivatives being implicated as hormone disruptors, there also exists the mechanism for bromine substituting for the methyl group in the nitrogenous base 5-methyluracil of DNA, creating the base-analog 5-bromouracil, which can be incorporated into DNA and induce a point mutation via base substitution.[14]
Metabolism
MDPV undergoes CYP450 2D6, 2C19 and COMT phase 1 metabolism (liver) into methylcatechol and pyrrolidine, which in turn are glucuronated (uridine 5'-diphospho-glucuronosyl-transferase) allowing it to be excreted by the kidneys, with only a small fraction of the metabolites being excreted into the fecal matter.[15] No free pyrrolidine will be detected in the urine.[16]
Molecularly, this is seen as demethylenation of methylenedioxypyrovalerone (CYP2D6), followed by methylation of the aromatic ring via catechol-O-methyl transferase. Then hydroxylation of both the aromatic ring and side chain takes place followed by and oxidation of the pyrimidine ring to the corresponding lactam, with subsequent detachment and ring opening to the corresponding carboxylic acid.[17]
Legality
In the UK, following the ACMD's report on cathinone derivatives,[12] MDPV is a Class B drug under the Misuse of Drugs Act 1971, making it illegal to sell, buy, or possess without a license. Penalties include a maximum of five years and/or unlimited fine for possession; up to 14 years and/or unlimited fine for production or trafficking. See list of drugs illegal in the UK for more information.
MDPV is specifically listed as a controlled substance in Finland (listed appendix IV substance as of 28 June 2010),[18] Denmark and Sweden. In Sweden a 33-year-old man has been sentenced to six years in prison by an appellate court, Hovrätt, for possession of 250 grams of MDPV that had been acquired prior to criminalization.[19]
United States
In the United States, MDPV is a DEA federally scheduled drug. On October 21, 2011, the DEA issued a temporary one year ban on MDPV, classifying it as a schedule I substance. Schedule I status is reserved for those substances with a high potential for abuse, no currently accepted use for treatment in the United States and a lack of accepted safety for use of the drug under medical supervision.[20][21]
Prior to the Federal ban being announced, it was already banned in in Louisiana[1] and Florida.[22][23][24][25] On March 24, 2011, Kentucky passed bill HB 121 which makes MDPV, as well as three other cathinones, controlled substances in the state. It also makes it a Class A misdemeanor to sell the drug, and a Class B misdemeanor to possess it.[26]
MDPV is banned in New Jersey under Pamela's Law. The law is named after Pamela Schmidt, a Rutgers University student, murdered in March 2011 by an alleged user of MDPV.[27]
As of April 15, 2011, two of the chemicals used in making MDPV had been banned in seven states, including Louisiana, Florida, Alabama, Mississippi, North Dakota, Washington and New Jersey. One of the chemicals used in MDPV has been banned in 10 more states, including, Idaho, Utah, Wyoming, New Mexico, Arkansas, Kentucky, Michigan, Virgina, West Virginia and North Carolina.[28] The New Jersey Division of Consumer Affairs made it "a crime to [knowingly] manufacture, distribute, sell, or possess designer drugs labeled as 'bath salts'"[29][30]
On May 5, 2011, Tennessee Governor Bill Haslam signed a law making it a crime to knowingly produce, manufacture, distribute, sell, offer for sale or possess with intent to produce, manufacture, distribute, sell, or offer for sale any product containing 3,4-Methylenedioxypyrovalerone (MDPV).[31]
On July 6, 2011, the governor of Maine signed a bill establishing fines for possession and penalties for trafficking of MDPV.[32]
On September 7, 2011, the DEA is taking advantage of its emergency scheduling authority to ban Mephedrone (4-MMC), MDPV, and Methylone (M1). The substances will be illegal to possess and sell for 12 months until the DEA and Department of Health and Human Services (DHS) determines if these substances should be permanently banned.[citation needed]
On October 17, 2011, an Ohio law banning Synthetic Drugs took effect barring selling and/or possession of "any material, compound, mixture, or preparation that contains any quantity of the following substances having a stimulant effect on the central nervous system, including their salts, isomers, and salts of isomers" listing (1) ephedrine and (2) pyrovalerone. It also includes "Methyenedioxypyrovalerone" Methylenedioxypyrovalerone, or MDPV (as described in this article) is unaffected by this law as it is (1) not mentioned and (2) chemically bonded to a pyrovalerone group/(as opposed to a mixture, compound or complex containing pyrovalerone.) It is currently legal to own, sell, purchase and possess. Possession of the fictional substance "Methyenedioxypyrovalerone" is a misdemeanor, while selling is a felony, inventing it may carry harsher penalties.[33] [34] Four days after this Ohio law went into effect the DEA's national emergency ban was implemented.[citation needed]
Documented Misuse
In April 2011, two weeks after they went missing, two men in Warren County, Pennsylvania, were found dead in the Allegheny National Forest. The official cause of death of both men was hypothermia, but toxicology reports later confirmed that both Troy Johnson, 29, and Terry Sumrow, 28, had ingested MDPV shortly before their deaths. "It wasn't anything to kill them, but enough to get them messed up," Warren County coroner Jeremiah Borden said. MDPV containers were found in their vehicle along with spoons, hypodermic syringes and marijuana paraphernalia.[35] In April 2011, an Alton, Illinois, woman apparently died from an MDPV overdose.[36] In May 2011, The CDC reported on Emergency Department Visits After Use of "Bath Salts" in Michigan. One person was reported dead on arrival at the ED. Associates of the dead person reported he had used bath salts. His toxicology results revealed high levels of MDPV in addition to marijuana and prescription drugs. The primary factor contributing to death was cited as MDPV toxicity after autopsy was performed.[37]
Treatment
Anyone suspected of having taken MDPV and needing possible treatment should be immediately referred to their personal physician or the nearest poison control center.[38] Physicians often treat MDPV overdose cases with anxiolytics, such as benzodiazepines, to lessen the drug-induced activity in the brain and body.[39] In some cases, general anesthesia was used due to sedatives being ineffective [40]
Treatment in the emergency department for severe hypertension, tachycardia, agitation, or seizures consists of large doses of lorazepam in 2 - 4 mg increments every 10–15 minutes intravenously or intramuscularly. If this is not effective, haloperidol is an alternative treatment. It has been found that the use of any beta blockers to treat hypertension in these patients can cause an unopposed peripheral alpha-adrenergic effect with a dangerous paradoxical rise in blood pressure.[41]
References
- ^ a b c d e f g h i j k l http://194.83.136.209/documents/reports/MDPV.pdf. MDPV report, Psychonaut Research Web Mapping Project
- ^ http://www.kmbc.com/news/26256067/detail.html. Abuse Of Fake 'Bath Salts' Sends Dozens To ER in gas stations and convenience stores, similar to the marketing for Spice and K2 as incense.
- ^ http://healthybodydaily.com/dr-oz-in-case-you-missed-it/dr-oz-bath-salts-mdpv-bath-salts-drug-over-the-counter. Dr. Oz: Bath Salts| MDPV Bath Salts Drug Over The Counter.
- ^ News Desk Manager, Samantha Morgan (9 November 2010). "Parents cautioned against over the counter synthetic speed". NBC 33 News. http://www.nbc33tv.com/consumer-alert/parents-cautioned-against-over-the-counter-synthetic-speed. Retrieved 16 May 2011.
- ^ Kelsey Scram (6 January 2011). "Bath Salts Used to Get High". NBC 33 News. http://www.nbc33tv.com/news/bath-salts-used-to-get-high. Retrieved 16 May 2011.
- ^ a b c Brandt, S.; (2010) "Analysis of NRG ‘legal highs’ in the UK: Identification and formation of novel cathinones". Drug Test. Analysis. doi: 10.1002/dta.204. PMID: 21191917
- ^ Hello and MDPV question - Bluelight
- ^ Westphal F, Junge T, Rösner P, Sönnichsen F, Schuster F (2009). "Mass and NMR spectroscopic characterization of 3,4-methylenedioxypyrovalerone: A designer drug with alpha-pyrrolidinophenone structure". Forensic Science International 190 (1–3): 1–8. doi:10.1016/j.forsciint.2009.05.001. PMID 19500924.
- ^ 1-[(3,4-Methylenedioxy)phenyl]-2-pyrrolidino-1-alkanones as stimulants. (Boehringer Ingelheim G.m.b.H.). Brit. (1969), 7 pp. CODEN: BRXXAA GB 1149366 19690423 Patent. Priority: DE 19650523. CAN 72:21608 AN 1970:21608 CAPLUS
- ^ a b c d e f http://www.deadiversion.usdoj.gov/drugs_concern/mdpv.pdf DEA report on MDPV
- ^ Insid
- ^ a b http://www.namsdl.org/documents/ACMDCathinonesReport.pdf Advisory Council on the Misuse of Drugs - Consideration of the cathinones
- ^ Loo, P; 2010 Jun. 3. "Convenient Synthesis and Spectroscopic Data of Methcathinone Analogs". 4th Seminar of European Customs Chemists.
- ^ Nester EW, Anderson DG and Roberts CE, Jr. (4 June 2009). Loose Leaf Version of Microbiology: A Human Perspective. McGraw-Hill. ISBN 9780077366476. http://books.google.com/books?id=rbl9PgAACAAJ. Retrieved 25 June 2011.
- ^ Strano-Rossi, Sabina; Cadwallader, Amy; De La Torre, Xavier; Botrè, Francesco (September 30, 2010). "Toxicological determination and in vitro metabolism of the designer drug methylenedioxypyrovalerone (MPDV) by gas chromatography/mass spectrometry and liquid chromatography/quadrupole time-of-flight mass spectrometry". Rapid Communications in Mass Spectrometry 24 (18): 2706–2714. doi:10.1002/rcm.4692. PMID 20814976.
- ^ Michaelis, W; (1970) "The metabolism of pyrovalerone hydrochloride". J Med Chem. 13(3): 497-503.
- ^ Meyer, Markus; Du, Peng; Schuster, Frank; Maurer, Hans (December 2010). "Studies on the metabolism of the α-pyrrolidinophenone designer drug methylenedioxy-pyrovalerone (MDPV) in rat and human urine and human liver microsomes using GC–MS and LC–high-resolution MS and its detectability in urine by GC–MS". Journal of Mass Spectrometry 45 (12): 1426–1442. doi:10.1002/jms.1859. PMID 21053377.
- ^ Suomen valtioneuvosto (28. June 2010). "Finlex: huumausaineina pidettävistä aineista, valmisteista ja kasveista annetun valtioneuvoston asetuksen liitteen IV muuttamisesta" (in Finnish). Oikeusministeriö. Oikeusministeriö. http://www.finlex.fi/fi/laki/alkup/2010/20100596. Retrieved 25 January 2011.
- ^ "Hovrätten skärper straff i MDPV-dom" (in Swedish). Norrköpings Tidningar. June 4, 2010. http://www.nt.se/nyheter/artikel.aspx?articleid=6057819. Retrieved June 12, 2010.
- ^ DEA Press release (Oct 21, 2011). "Chemicals Used in "Bath Salts” Now Under Federal Control and Regulation". http://www.justice.gov/dea/pubs/pressrel/pr102111.html. Retrieved 2011-10-22.
- ^ Harris, Elizabeth (Oct 22, 2011). ["http://www.nytimes.com/2011/10/22/us/dea-bans-chemicals-used-in-bath-salts.html?hp "D.E.A. Bans Chemicals Used in ‘Bath Salts’"]. NY Times . "http://www.nytimes.com/2011/10/22/us/dea-bans-chemicals-used-in-bath-salts.html?hp. Retrieved 2011-10-22.
- ^ Rakow, Erica (Jan 26, 2011). "Florida makees sales and possession of bath salts illegal". WJHG-TV. http://www.wjhg.com/home/headlines/Florida_makes_sales_and_possession_of_bath_salts_illegal_114681959.html. Retrieved 2011-01-27.
- ^ Williams, Tyana (Jan 06, 2011). "Livingston deputies begin seizing "bath salts". WAFB. http://www.wafb.com/Global/story.asp?S=13798678. Retrieved 2011-01-08.
- ^ McConnaughey, Janet (2010-12-23). "Drugs disguised as bath salts send users to ERs". WAFB. Associated Press. http://www.msnbc.msn.com/id/40797021/ns/health-addictions/. Retrieved 2011-01-08.
- ^ Allen, Greg (February 8, 2011). "Florida Bans Cocaine-Like 'Bath Salts' Sold In Stores". NPR. http://www.npr.org/2011/02/08/133399834/florida-bans-cocaine-like-bath-salts-sold-in-stores. Retrieved May 22, 2011.
- ^ Beshear, Steve. "Press Release". Gov. Beshear signs law banning new synthetic drugs. Commonwealth of Kentucky. http://www.governor.ky.gov/pressrelease.htm?PostingGUID={D5D7C9CB-DDE8-4581-A6A9-40DA3FC6E877}. Retrieved Wednesday, March 23, 2011.
- ^ "Governor bans bath salts after student’s death". Daily Targum. September 2, 2011. http://www.dailytargum.com/news/governor-bans-bath-salts-after-student-s-death-1.2618943. Retrieved 2011-09-03. "Gov. Chris Christie signed "Pamela's Law" into legislation last week, which will ban the sale, possession and use of bath salts, a synthetic drug that affects users in a similar way to methamphetamines, in New Jersey. The law is named after Pamela Schmidt, a University student who was murdered in March. Authorities believe her boyfriend William Parisio Jr., who was under the influence of bath salts at the time of her murder, to be the suspect. ..."
- ^ April 28, 2011. "So-called BATH SALTS drugs: Nationwide Efforts" New Jersey Department of Consumer Affairs.
- ^ New Jersey Division of Consumer Affairs. "WARNING: Designer Drugs Labeled as Bath Salts"
- ^ The New Jersey code of criminal justice. "New Jersey Statutes - Title 2C The New Jersey Code of Criminal Justice - 2C:35-5 Manufacturing, distributing or dispensing"
- ^ State of Tennessee, 2011. "HOUSE BILL NO. 457, PUBLIC CHAPTER NO. 169. 39-17-452"
- ^ "New law sets fine at $350 for ‘bath salts’ possession". Portland Press Herald. 7 July 2011. http://www.pressherald.com/news/StateLocal-Dispatches-2011-07-07.html. Retrieved 7 July 2011.
- ^ Ohio's Amendment to Controlled Substances act bans non-existent "substance" due to mispelling[sic] and lack of science [1]
- ^ WBNS-TV Columbus Oct. 17, 2011 Synthetic Drugs Ban Goes into effect [2]"Synthetic Drug Ban goes into effect"]
- ^ Wells, Dean (April 9. 2011). "Dead men had used bath salts". Warren Times Observer. http://www.timesobserver.com/page/content.detail/id/545684/Report--Dead-men-had-used-bath-salts.html?nav=5006. Retrieved May 22, 2011.
- ^ Wilson, Todd (May 12, 2011). "Illinois lawmakers target bath salts used as a drug". Chicago Tribune. http://www.chicagotribune.com/health/ct-met-bath-salts-ban-20110512,0,3367566.story. Retrieved May 22, 2011.
- ^ Centers for Disease Control and Prevention (CDC) (May 20, 2011). "Emergency Department Visits After Use of a Drug Sold as "Bath Salts"-Michigan, November 13, 2010-March 31, 2011". Morbidity and Mortality Weekly Report 60 (19): 624–627. PMID 21597456
- ^ Oregon Health & Science University, Bath Salts Fast Facts, March 25, 2011, http://www.ohsu.edu/poison/documents/FFBath_Salts.pdf?WT_rank=2
- ^ Salter, Jim; Suhr, Jim (April 7, 2011). "AP IMPACT: Synthetic drugs send thousands to ER". Bloomberg Businessweek. http://www.businessweek.com/ap/financialnews/D9MEUIRO0.htm. Retrieved May 22, 2011.
- ^ Goodnough, Abby; Zezima, Katie (July 16, 2011). "An Alarming New Stimulant, Sold Legally in Many States". The New York Times. http://www.nytimes.com/2011/07/17/us/17salts.html.
- ^ "Bath Salts Healthcare Provider Fact Sheet". Feb 3, 2011. http://www.bhsj.org/Publications/faqs/FAQ-BathSalts-MDCH.pdf.
External links
- Pubchem - similar compounds
- Meltzer PC, Butler D, Deschamps JR, Madras BK (February 2006). "1-(4-Methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one (Pyrovalerone) analogs. A promising class of monoamine uptake inhibitors". J. Med. Chem. 49 (4): 1420–32. doi:10.1021/jm050797a. PMC 2602954. PMID 16480278. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2602954.
- Erowid MDPV Vault
- MDPV report Psychonaut Research Web Mapping Project
- ChemSub Online: Methylenedioxypyrovalerone.
Stimulants (N06B) Adamantanes Adaphenoxate • Adapromine • Amantadine • Bromantane • Chlodantane • Gludantane • Memantine • Midantane
Adenosine antagonists 8-Chlorotheophylline • 8-Cyclopentyltheophylline • 8-Phenyltheophylline • Aminophylline • Caffeine • CGS-15943 • Dimethazan • Paraxanthine • SCH-58261 • Theobromine • TheophyllineAlkylamines Arylcyclohexylamines Benocyclidine • Dieticyclidine • Esketamine • Eticyclidine • Gacyclidine • Ketamine • Phencyclamine • Phencyclidine • Rolicyclidine • Tenocyclidine • Tiletamine
Benzazepines 6-Br-APB • SKF-77434 • SKF-81297 • SKF-82958
Cholinergics A-84543 • A-366,833 • ABT-202 • ABT-418 • AR-R17779 • Altinicline • Anabasine • Arecoline • Cotinine • Cytisine • Dianicline • Epibatidine • Epiboxidine • GTS-21 • Ispronicline • Nicotine • PHA-543,613 • PNU-120,596 • PNU-282,987 • Pozanicline • Rivanicline • Sazetidine A • SIB-1553A • SSR-180,711 • TC-1698 • TC-1827 • TC-2216 • TC-5619 • Tebanicline • UB-165 • Varenicline • WAY-317,538
Convulsants Anatoxin-a • Bicuculline • DMCM • Flurothyl • Gabazine • Pentetrazol • Picrotoxin • Strychnine • Thujone
Eugeroics Adrafinil • Armodafinil • CRL-40941 • Modafinil
Oxazolines 4-Methylaminorex • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone
Phenethylamines 1-(4-Methylphenyl)-2-aminobutane • 1-Phenyl-2-(piperidin-1-yl)pentan-3-one • 1-Methylamino-1-(3,4-methylenedioxyphenyl)propane • 2-Fluoroamphetamine • 2-Fluoromethamphetamine • 2-OH-PEA • 2-Phenyl-3-aminobutane • 2-Phenyl-3-methylaminobutane • 2,3-MDA • 3-Fluoroamphetamine • 3-Fluoroethamphetamine • 3-Fluoromethcathinone • 3-Methoxyamphetamine • 3-Methylamphetamine • 3,4-DMMC • 4-BMC • 4-Ethylamphetamine • 4-FA • 4-FMA • 4-MA • 4-MMA • 4-MTA • 6-FNE • Alfetamine • α-Ethylphenethylamine • Amfecloral • Amfepentorex • Amfepramone • Amidephrine • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • Arbutamine • β-Methylphenethylamine • β-Phenylmethamphetamine • Benfluorex • Benzedrone • Benzphetamine • BDB (J) • BOH (Hydroxy-J) • BPAP • Buphedrone • Bupropion (Amfebutamone) • Butylone • Cathine • Cathinone • Chlorphentermine • Cinnamedrine • Clenbuterol • Clobenzorex • Cloforex • Clortermine • D-Deprenyl • Denopamine • Dimethoxyamphetamine • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, Metamfepramone) • Dobutamine • DOPA (Dextrodopa, Levodopa) • Dopamine • Dopexamine • Droxidopa • EBDB (Ethyl-J) • Ephedrine • Epinephrine (Adrenaline) • Epinine (Deoxyepinephrine) • Etafedrine • Ethcathinone (Ethylpropion) • Ethylamphetamine (Etilamfetamine) • Ethylnorepinephrine (Butanefrine) • Ethylone • Etilefrine • Famprofazone • Fenbutrazate • Fencamine • Fenethylline • Fenfluramine (Dexfenfluramine) • Fenmetramide • Fenproporex • Flephedrone • Fludorex • Furfenorex • Gepefrine • HMMA • Hordenine • Ibopamine • IMP • Indanylamphetamine • Isoetarine • Isoethcathinone • Isoprenaline (Isoproterenol) • L-Deprenyl (Selegiline) • Lefetamine • Lisdexamfetamine • Lophophine (Homomyristicylamine) • Manifaxine • MBDB (Methyl-J; "Eden") • MDA (Tenamfetamine) • MDBU • MDEA ("Eve") • MDMA ("Ecstasy", "Adam") • MDMPEA (Homarylamine) • MDOH • MDPR • MDPEA (Homopiperonylamine) • Mefenorex • Mephedrone • Mephentermine • Metanephrine • Metaraminol • Methamphetamine (Desoxyephedrine, Methedrine; Dextromethamphetamine, Levomethamphetamine) • Methoxamine • Methoxyphenamine • MMA • Methcathinone (Methylpropion) • Methedrone • Methoxyphenamine • Methylone • MMDA • MMDMA • MMMA • Morazone • N-Benzyl-1-phenethylamine • N,N-Dimethylphenethylamine • Naphthylamphetamine • Nisoxetine • Norepinephrine (Noradrenaline) • Norfenefrine • Norfenfluramine • Normetanephrine • Octopamine • Orciprenaline • Ortetamine • Oxilofrine • Paredrine (Norpholedrine, Oxamphetamine, Mycadrine) • PBA • PCA • PHA • Pargyline • Pentorex (Phenpentermine) • Pentylone • Phendimetrazine • Phenmetrazine • Phenpromethamine • Phentermine • Phenylalanine • Phenylephrine (Neosynephrine) • Phenylpropanolamine • Pholedrine • PIA • PMA • PMEA • PMMA • PPAP • Prenylamine • Propylamphetamine • Pseudoephedrine • Radafaxine • Ropinirole • Salbutamol (Albuterol; Levosalbutamol) • Sibutramine • Synephrine (Oxedrine) • Theodrenaline • Tiflorex (Flutiorex) • Tranylcypromine • Tyramine • Tyrosine • Xamoterol • Xylopropamine • Zylofuramine
Piperazines Piperidines 1-Benzyl-4-(2-(diphenylmethoxy)ethyl)piperidine • 1-(3,4-Dichlorophenyl)-1-(piperidin-2-yl)butane • 2-Benzylpiperidine • 2-Methyl-3-phenylpiperidine • 3,4-Dichloromethylphenidate • 4-Benzylpiperidine • 4-Methylmethylphenidate • Desoxypipradrol • Difemetorex • Diphenylpyraline • Ethylphenidate • Methylnaphthidate • Methylphenidate (Dexmethylphenidate) • N-Methyl-3β-propyl-4β-(4-chlorophenyl)piperidine • Nocaine • Phacetoperane • Pipradrol • SCH-5472
Pyrrolidines 2-Diphenylmethylpyrrolidine • α-PPP • α-PBP • α-PVP • Diphenylprolinol • MDPPP • MDPBP • MDPV • MPBP • MPHP • MPPP • MOPPP • Naphyrone • PEP • Prolintane • Pyrovalerone
Tropanes 3-CPMT • 3'-Chloro-3α-(diphenylmethoxy)tropane • 3-Pseudotropyl-4-fluorobenzoate • 4'-Fluorococaine • AHN-1055 • Altropane (IACFT) • Brasofensine • CFT (WIN 35,428) • β-CIT (RTI-55) • Cocaethylene • Cocaine • Dichloropane (RTI-111) • Difluoropine • FE-β-CPPIT • FP-β-CPPIT • Ioflupane (123I) • Norcocaine • PIT • PTT • RTI-31 • RTI-32 • RTI-51 • RTI-105 • RTI-112 • RTI-113 • RTI-117 • RTI-120 • RTI-121 (IPCIT) • RTI-126 • RTI-150 • RTI-154 • RTI-171 • RTI-177 • RTI-183 • RTI-193 • RTI-194 • RTI-199 • RTI-202 • RTI-204 • RTI-229 • RTI-241 • RTI-336 • RTI-354 • RTI-371 • RTI-386 • Salicylmethylecgonine • Tesofensine • Troparil (β-CPT, WIN 35,065-2) • Tropoxane • WF-23 • WF-33 • WF-60
Others 1-(Thiophen-2-yl)-2-aminopropane • 2-Amino-1,2-dihydronaphthalene • 2-Aminoindane • 2-Aminotetralin • 2-MDP • 2-Phenylcyclohexylamine • 2-Phenyl-3,6-dimethylmorpholine • 3-Benzhydrylmorpholine • 3,3-Diphenylcyclobutanamine • 5-(2-Aminopropyl)indole • 5-Iodo-2-aminoindane • AL-1095 • Amfonelic acid • Amineptine • Amiphenazole • Atipamezole • Atomoxetine (Tomoxetine) • Bemegride • Benzydamine • BTQ • BTS 74,398 • Carphedon • Ciclazindol • Cilobamine • Clofenciclan • Cropropamide • Crotetamide • Cypenamine • D-161 • Diclofensine • Dimethocaine • Efaroxan • Etamivan • EXP-561 • Fencamfamine • Fenpentadiol • Feprosidnine • G-130 • Gamfexine • Gilutensin • GSK1360707F • GYKI-52895 • Hexacyclonate • Idazoxan • Indanorex • Indatraline • JNJ-7925476 • JZ-IV-10 • Lazabemide • Leptacline • Levopropylhexedrine • Lomevactone • LR-5182 • Mazindol • Meclofenoxate • Medifoxamine • Mefexamide • Mesocarb • Methastyridone • Methiopropamine • N-Methyl-3-phenylnorbornan-2-amine • Nefopam • Nikethamide • Nomifensine • O-2172 • Oxaprotiline • Phthalimidopropiophenone • PNU-99,194 • Propylhexedrine • PRC200-SS • Rasagiline • Rauwolscine • Rubidium chloride • Setazindol • Tametraline • Tandamine • Trazium • UH-232 • Yohimbine
See also Sympathomimetic aminesAdrenergics Receptor ligands Agonists: 5-FNE • 6-FNE • Amidephrine • Anisodamine • Anisodine • Cirazoline • Dipivefrine • Dopamine • Ephedrine • Epinephrine (Adrenaline) • Etilefrine • Ethylnorepinephrine • Indanidine • Levonordefrin • Metaraminol • Methoxamine • Methyldopa • Midodrine • Naphazoline • Norepinephrine (Noradrenaline) • Octopamine • Oxymetazoline • Phenylephrine • Phenylpropanolamine • Pseudoephedrine • Synephrine • Tetrahydrozoline
Antagonists: Abanoquil • Adimolol • Ajmalicine • Alfuzosin • Amosulalol • Arotinolol • Atiprosin • Benoxathian • Buflomedil • Bunazosin • Carvedilol • CI-926 • Corynanthine • Dapiprazole • DL-017 • Domesticine • Doxazosin • Eugenodilol • Fenspiride • GYKI-12,743 • GYKI-16,084 • Indoramin • Ketanserin • L-765,314 • Labetalol • Mephendioxan • Metazosin • Monatepil • Moxisylyte (Thymoxamine) • Naftopidil • Nantenine • Neldazosin • Nicergoline • Niguldipine • Pelanserin • Phendioxan • Phenoxybenzamine • Phentolamine • Piperoxan • Prazosin • Quinazosin • Ritanserin • RS-97,078 • SGB-1,534 • Silodosin • SL-89.0591 • Spiperone • Talipexole • Tamsulosin • Terazosin • Tibalosin • Tiodazosin • Tipentosin • Tolazoline • Trimazosin • Upidosin • Urapidil • Zolertine
* Note that many TCAs, TeCAs, antipsychotics, ergolines, and some piperazines like buspirone, trazodone, nefazodone, etoperidone, and mepiprazole all antagonize α1-adrenergic receptors as well, which contributes to their side effects such as orthostatic hypotension.Agonists: (R)-3-Nitrobiphenyline • 4-NEMD • 6-FNE • Amitraz • Apraclonidine • Brimonidine • Cannabivarin • Clonidine • Detomidine • Dexmedetomidine • Dihydroergotamine • Dipivefrine • Dopamine • Ephedrine • Ergotamine • Epinephrine (Adrenaline) • Esproquin • Etilefrine • Ethylnorepinephrine • Guanabenz • Guanfacine • Guanoxabenz • Levonordefrin • Lofexidine • Medetomidine • Methyldopa • Mivazerol • Naphazoline • Norepinephrine (Noradrenaline) • Phenylpropanolamine • Piperoxan • Pseudoephedrine • Rilmenidine • Romifidine • Talipexole • Tetrahydrozoline • Tizanidine • Tolonidine • Urapidil • Xylazine • Xylometazoline
Antagonists: 1-PP • Adimolol • Aptazapine • Atipamezole • BRL-44408 • Buflomedil • Cirazoline • Efaroxan • Esmirtazapine • Fenmetozole • Fluparoxan • GYKI-12,743 • GYKI-16,084 • Idazoxan • Mianserin • Mirtazapine • MK-912 • NAN-190 • Olanzapine • Phentolamine • Phenoxybenzamine • Piperoxan • Piribedil • Rauwolscine • Rotigotine • SB-269,970 • Setiptiline • Spiroxatrine • Sunepitron • Tolazoline • Yohimbine
* Note that many atypical antipsychotics and azapirones like buspirone and gepirone (via metabolite 1-PP) antagonize α2-adrenergic receptors as well.βAgonists: 2-FNE • 5-FNE • Amibegron • Arbutamine • Arformoterol • Arotinolol • BAAM • Bambuterol • Befunolol • Bitolterol • Broxaterol • Buphenine • Carbuterol • Cimaterol • Clenbuterol • Denopamine • Deterenol • Dipivefrine • Dobutamine • Dopamine • Dopexamine • Ephedrine • Epinephrine (Adrenaline) • Etafedrine • Etilefrine • Ethylnorepinephrine • Fenoterol • Formoterol • Hexoprenaline • Higenamine • Indacaterol • Isoetarine • Isoprenaline (Isoproterenol) • Isoxsuprine • Labetalol • Levonordefrin • Levosalbutamol • Mabuterol • Methoxyphenamine • Methyldopa • Norepinephrine (Noradrenaline) • Orciprenaline • Oxyfedrine • Phenylpropanolamine • Pirbuterol • Prenalterol • Ractopamine • Procaterol • Pseudoephedrine • Reproterol • Rimiterol • Ritodrine • Salbutamol (Albuterol) • Salmeterol • Solabegron • Terbutaline • Tretoquinol • Tulobuterol • Xamoterol • Zilpaterol • Zinterol
Antagonists: Acebutolol • Adaprolol • Adimolol • Afurolol • Alprenolol • Alprenoxime • Amosulalol • Ancarolol • Arnolol • Arotinolol • Atenolol • Befunolol • Betaxolol • Bevantolol • Bisoprolol • Bopindolol • Bormetolol • Bornaprolol • Brefonalol • Bucindolol • Bucumolol • Bufetolol • Buftiralol • Bufuralol • Bunitrolol • Bunolol • Bupranolol • Burocrolol • Butaxamine • Butidrine • Butofilolol • Capsinolol • Carazolol • Carpindolol • Carteolol • Carvedilol • Celiprolol • Cetamolol • Cicloprolol • Cinamolol • Cloranolol • Cyanopindolol • Dalbraminol • Dexpropranolol • Diacetolol • Dichloroisoprenaline • Dihydroalprenolol • Dilevalol • Diprafenone • Draquinolol • Dropranolol • Ecastolol • Epanolol • Ericolol • Ersentilide • Esatenolol • Esmolol • Esprolol • Eugenodilol • Exaprolol • Falintolol • Flestolol • Flusoxolol • Hydroxycarteolol • Hydroxytertatolol • ICI-118,551 • Idropranolol • Indenolol • Indopanolol • Iodocyanopindolol • Iprocrolol • Isoxaprolol • Isamoltane • Labetalol • Landiolol • Levobetaxolol • Levobunolol • Levocicloprolol • Levomoprolol • Medroxalol • Mepindolol • Metalol • Metipranolol • Metoprolol • Moprolol • Nadolol • Nadoxolol • Nafetolol • Nebivolol • Neraminol • Nifenalol • Nipradilol • Oberadilol • Oxprenolol • Pacrinolol • Pafenolol • Pamatolol • Pargolol • Parodilol • Penbutolol • Penirolol • PhQA-33 • Pindolol • Pirepolol • Practolol • Primidolol • Procinolol • Pronethalol • Propafenone • Propranolol • Ridazolol • Ronactolol • Soquinolol • Sotalol • Spirendolol • SR 59230A • Sulfinalol • TA-2005 • Talinolol • Tazolol • Teoprolol • Tertatolol • Terthianolol • Tienoxolol • Tilisolol • Timolol • Tiprenolol • Tolamolol • Toliprolol • Tribendilol • Trigevolol • Xibenolol • XipranololReuptake inhibitors Selective norepinephrine reuptake inhibitors: Amedalin • Atomoxetine (Tomoxetine) • Ciclazindol • Daledalin • Esreboxetine • Lortalamine • Mazindol • Nisoxetine • Reboxetine • Talopram • Talsupram • Tandamine • Viloxazine; Norepinephrine-dopamine reuptake inhibitors: Amineptine • Bupropion (Amfebutamone) • Fencamine • Fencamfamine • Lefetamine • Levophacetoperane • LR-5182 • Manifaxine • Methylphenidate • Nomifensine • O-2172 • Radafaxine; Serotonin-norepinephrine reuptake inhibitors: Bicifadine • Desvenlafaxine • Duloxetine • Eclanamine • Levomilnacipran • Milnacipran • Sibutramine • Venlafaxine; Serotonin-norepinephrine-dopamine reuptake inhibitors: Brasofensine • Diclofensine • DOV-102,677 • DOV-21,947 • DOV-216,303 • JNJ-7925476 • JZ-IV-10 • Methylnaphthidate • Naphyrone • NS-2359 • PRC200-SS • SEP-225,289 • SEP-227,162 • Tesofensine; Tricyclic antidepressants: Amitriptyline • Butriptyline • Cianopramine • Clomipramine • Desipramine • Dosulepin • Doxepin • Imipramine • Lofepramine • melitracen • Nortriptyline • Protriptyline • Trimipramine; Tetracyclic antidepressants: Amoxapine • Maprotiline • Mianserin • Oxaprotiline • Setiptiline; Others: Cocaine • CP-39,332 • EXP-561 • Fezolamine • Ginkgo biloba • Indeloxazine • Nefazodone • Nefopam • Pridefrine • Tapentadol • Teniloxazine • Tramadol • ZiprasidoneEnzyme inhibitors 3,4-DihydroxystyreneDBHCGS-19281A • SKF-64139 • SKF-7698Nonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima; MAO-B selective: D-Deprenyl • Selegiline (L-Deprenyl) • Ladostigil • Lazabemide • Milacemide • Mofegiline • Pargyline • Rasagiline • Safinamide
* Note that MAO-B inhibitors also influence norepinephrine/epinephrine levels since they inhibit the breakdown of their precursor dopamine.COMTOthers Ferrous Iron (Fe2+) • S-Adenosyl-L-Methionine • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal Phosphate) • Vitamin B9 (Folic acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersActivity enhancers: BPAP • PPAP; Release blockers: Bethanidine • Bretylium • Guanadrel • Guanazodine • Guanclofine • Guanethidine • Guanoxan; Toxins: Oxidopamine (6-Hydroxydopamine)List of adrenergic drugsDopaminergics Reuptake inhibitors PlasmalemmalDAT inhibitorsPiperazines: DBL-583 • GBR-12,935 • Nefazodone • Vanoxerine; Piperidines: BTCP • Desoxypipradrol • Dextromethylphenidate • Difemetorex • Ethylphenidate • Methylnaphthidate • Methylphenidate • Phencyclidine • Pipradrol; Pyrrolidines: Diphenylprolinol • Methylenedioxypyrovalerone (MDPV) • Naphyrone • Prolintane • Pyrovalerone; Tropanes: β-CPPIT • Altropane • Brasofensine • CFT • Cocaine • Dichloropane • Difluoropine • FE-β-CPPIT • FP-β-CPPIT • Ioflupane (123I) • Iometopane • RTI-112 • RTI-113 • RTI-121 • RTI-126 • RTI-150 • RTI-177 • RTI-229 • RTI-336 • Tenocyclidine • Tesofensine • Troparil • Tropoxane • WF-11 • WF-23 • WF-31 • WF-33; Others: Adrafinil • Armodafinil • Amfonelic acid • Amineptine • Benzatropine (Benztropine) • Bromantane • BTQ • BTS-74,398 • Bupropion (Amfebutamone) • Ciclazindol • Diclofensine • Dimethocaine • Diphenylpyraline • Dizocilpine • DOV-102,677 • DOV-21,947 • DOV-216,303 • Etybenzatropine (Ethylbenztropine) • EXP-561 • Fencamine • Fencamfamine • Fezolamine • GYKI-52,895 • Indatraline • Ketamine • Lefetamine • Levophacetoperane • LR-5182 • Manifaxine • Mazindol • Medifoxamine • Mesocarb • Modafinil • Nefopam • Nomifensine • NS-2359 • O-2172 • Pridefrine • Propylamphetamine • Radafaxine • SEP-225,289 • SEP-227,162 • Sertraline • Sibutramine • Tametraline • TripelennamineVMAT inhibitorsReleasing agents Morpholines: Fenbutrazate • Morazone • Phendimetrazine • Phenmetrazine; Oxazolines: 4-Methylaminorex (4-MAR, 4-MAX) • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone; Phenethylamines (also amphetamines, cathinones, phentermines, etc): 2-Hydroxyphenethylamine (2-OH-PEA) • 4-CAB • 4-Methylamphetamine (4-MA) • 4-Methylmethamphetamine (4-MMA) • Alfetamine • Amfecloral • Amfepentorex • Amfepramone • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • β-Methylphenethylamine (β-Me-PEA) • Benzodioxolylbutanamine (BDB) • Benzodioxolylhydroxybutanamine (BOH) • Benzphetamine • Buphedrone • Butylone • Cathine • Cathinone • Clobenzorex • Clortermine • D-Deprenyl • Dimethoxyamphetamine (DMA) • Dimethoxymethamphetamine (DMMA) • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, metamfepramone) • Ethcathinone (Ethylpropion) • Ethylamphetamine • Ethylbenzodioxolylbutanamine (EBDB) • Ethylone • Famprofazone • Fenethylline • Fenproporex • Flephedrone • Fludorex • Furfenorex • Hordenine • Lophophine (Homomyristicylamine) • Mefenorex • Mephedrone • Methamphetamine (Desoxyephedrine, Methedrine; Dextromethamphetamine, Levomethamphetamine) • Methcathinone (Methylpropion) • Methedrone • Methoxymethylenedioxyamphetamine (MMDA) • Methoxymethylenedioxymethamphetamine (MMDMA) • Methylbenzodioxolylbutanamine (MBDB) • Methylenedioxyamphetamine (MDA, tenamfetamine) • Methylenedioxyethylamphetamine (MDEA) • Methylenedioxyhydroxyamphetamine (MDOH) • Methylenedioxymethamphetamine (MDMA) • Methylenedioxymethylphenethylamine (MDMPEA, homarylamine) • Methylenedioxyphenethylamine (MDPEA, homopiperonylamine) • Methylone • Ortetamine • Parabromoamphetamine (PBA) • Parachloroamphetamine (PCA) • Parafluoroamphetamine (PFA) • Parafluoromethamphetamine (PFMA) • Parahydroxyamphetamine (PHA) • Paraiodoamphetamine (PIA) • Paredrine (Norpholedrine, Oxamphetamine) • Phenethylamine (PEA) • Pholedrine • Phenpromethamine • Prenylamine • Propylamphetamine • Tiflorex (Flutiorex) • Tyramine (TRA) • Xylopropamine • Zylofuramine; Piperazines: 2,5-Dimethoxy-4-bromobenzylpiperazine (2C-B-BZP) • Benzylpiperazine (BZP) • Methoxyphenylpiperazine (MeOPP, paraperazine) • Methylbenzylpiperazine (MBZP) • Methylenedioxybenzylpiperazine (MDBZP, piperonylpiperazine); Others: 2-Amino-1,2-dihydronaphthalene (2-ADN) • 2-Aminoindane (2-AI) • 2-Aminotetralin (2-AT) • 4-Benzylpiperidine (4-BP) • 5-IAI • Clofenciclan • Cyclopentamine • Cypenamine • Cyprodenate • Feprosidnine • Gilutensin • Heptaminol • Hexacyclonate • Indanylaminopropane (IAP) • Indanorex • Isometheptene • Methylhexanamine • Naphthylaminopropane (NAP) • Octodrine • Phthalimidopropiophenone • Propylhexedrine (Levopropylhexedrine) • Tuaminoheptane (Tuamine)Enzyme inhibitors PAH inhibitors3,4-DihydroxystyreneTH inhibitorsNonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima; MAO-B selective: D-Deprenyl • L-Deprenyl (Selegiline) • Ladostigil • Lazabemide • Milacemide • Pargyline • Rasagiline • SafinamideDBH inhibitorsOthers L-Phenylalanine → L-Tyrosine → L-DOPA (Levodopa)Ferrous iron (Fe2+) • Tetrahydrobiopterin • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal phosphate) • Vitamin B9 (Folic acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersActivity enhancers: Benzofuranylpropylaminopentane (BPAP) • Phenylpropylaminopentane (PPAP); Toxins: Oxidopamine (6-Hydroxydopamine)List of dopaminergic drugsCategories:- Aromatic ketones
- Benzodioxoles
- Cathinones
- Norepinephrine-dopamine reuptake inhibitors
- Pyrrolidinophenones
Wikimedia Foundation. 2010.
