- Conhydrine
-
Conhydrine  2-α-Hydroxypropyl-piperidine
2-α-Hydroxypropyl-piperidineIdentifiers ChemSpider 9919452 
Jmol-3D images Image 1 - O[C@H](CC)[C@H]1NCCCC1
Properties Molecular formula C8H17NO Molar mass 143.23 g mol−1 Melting point 121 °C, 394 K, 250 °F
Boiling point 226 °C, 499 K, 439 °F
Solubility in water moderate Solubility in ethanol good Solubility in chloroform good Solubility in diethylether moderate Chiral rotation [α]D +10° (natural)  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Conhydrine is a poisonous alkaloid found in poison hemlock in small quantities.
Isolation and properties
This oxygenated alkaloid was isolated by Wertheim.[1] It crystallises in colourless leaflets, has a coniine-like odour, can be sublimed, and is strongly basic. It crystallises readily from ether. The salts are crystalline; the aurichloride small rhombs or prisms, mp. 133 °C; the benzoyl derivative mp. 132 °C.
Constitution
On oxidation with chromic acid, conhydrine yields L-piperidyl-2-carboxylic acid.[2] It is converted into L-coniine either by reduction of the iodo-derivative (iodoconiine), C8H16IN, formed by the action of hydriodic acid and phosphorus at 180 °C[3][4][5] or by hydrogenation of the mixture of coniceines produced, when it is dehydrated by phosphorus pentoxide in toluene.[6]
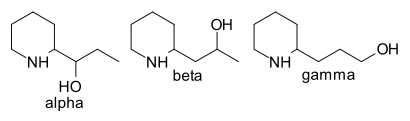
These and other observations indicate that the oxygen atom must occur as a hydroxyl group, in the n-propyl side chain in either the α- or β-position, since the γ-position would involve the production of piperidyl-2-propionic acid on oxidation. 2-β-Hydroxypropyl-piperidine suggested by Willstätter[7] seemed to be excluded, since neither of the two forms of this product prepared by Löffler and Tschunke[8] resembled conhydrine, and these authors suggested the α-position as probably representing the alkaloid. Support for this view was provided by Hess and coworkers[9][10][11] [12][13], who showed that DL--N-methylconhydrone is N-methyl-2-piperidyl ethyl ketone, that DL-conhydrine (mp. 69-70 °C), produced by a somewhat indirect method, is identical with the product, mp. 69.5-71.5 °C, prepared by Engler and Bauer[14][15] by the reduction with sodium in ethyl alcohol of 2-pyridyl ethyl ketone, and that conhydrine on dehydrogenation over platinum or palladium asbestos gives rise to a mixture of tetrahydropyridyl 2-ethyl ketone and 2-α-hydroxypropyl-pyridine. Späth and Adler[6] have shown that conhydrine can be degraded in two stages by exhaustive methylation to trimethylamine, and a mixture of two products, an oil, C8H14O, bp. 157-9 °C@744 mmHg, and a crystalline substance, C8H16O2, mp. 75-6 °C. The oil, when heated with water at 170 °C is converted, by addition of a molecule of water, into the crystalline substance. The latter contains two active hydrogen atoms (Zerewitinoff estimation), and on exposure to hydrogen over Pd/C absorbs enough to saturate one double bond producing a new substance, mp. 94-6 °C. On oxidation with permanganate in dilute sulfuric acid, propionaldehyde and succinic acid are produced, whilst the saturated substance, mp. 94-6 °C, is oxidised to n-valeric acid. These results indicate that the substance of mp. 75-6 °C is εζ-dihydroxy-Δα-n-octene, that the oil, C8H14O, is the corresponding oxide, and that the representation of conhydrine as 2-α-hydroxypropyl-pyridine accounts for their production.
References
- ^ Wertheim, Th. (1856). "Ueber ein neues Alkaloïd in Conium maculatum". Annalen der Chemie und Pharmacie 100 (3): 328. doi:10.1002/jlac.18561000311.
- ^ Willstätter, Richard (1901). "Oxydation des Conydrins". Berichte der deutschen chemischen Gesellschaft 34 (2): 3166. doi:10.1002/cber.190103402290.
- ^ Hofmann, A. W. (1885). "Zur Kenntniss der Coniin-Gruppe". Berichte der deutschen chemischen Gesellschaft 18: 5. doi:10.1002/cber.18850180103.
- ^ Lellmann, Eugen (1890). "Ueber die Coniceïne". Justus Liebig's Annalen der Chemie 259 (2–3): 193. doi:10.1002/jlac.18902590205.
- ^ Löffer, Karl; Friedrich, Gotthold (1909). "Die Synthese des β-Coniceins (l-α-Allyl-piperidin)". Berichte der deutschen chemischen Gesellschaft 42: 107. doi:10.1002/cber.19090420113.
- ^ a b Späth, Ernst; Adler, Erich (1933). "Zur Konstitution des Konhydrins". Monatshefte für Chemie 63: 127. doi:10.1007/BF01522210.
- ^ Willstätter, Richard (1901). "Oxydation des Conydrins". Berichte der deutschen chemischen Gesellschaft 34 (2): 3166. doi:10.1002/cber.190103402290.
- ^ Löffler, Karl; Tschunke, Gotthold (1909). "Über die Konstitution des Conhydrins (optisch-aktives α-Äthyl-piperidyl-alkin)". Berichte der deutschen chemischen Gesellschaft 42: 929. doi:10.1002/cber.190904201153.
- ^ Hess, Kurt; Eichel, Annaliese (1917). "Über die Alkaloide des Granatapfelbaumes. IV. Ein Trennungsgang für die Reindarstellung der Pelletierin-Alkaloide. Aufklärung der Konstitution des Methyl-isopelletierins (Methyl-pelletierin, Isomethyl-pelletierin). Umwandlung des Conhydrins in Methylisopelletierin. Die Konstitution des Conhydrins". Berichte der deutschen chemischen Gesellschaft 50 (2): 1386. doi:10.1002/cber.19170500239.
- ^ Heß, Kurt (1919). "Über die Beziehung von Methyl-isopelletierin,d,l-Methyl-conhydrinon und (N-Methyl-piperidyl)-propan-1-on. Ein Isomeriefall von Verbindungen mit einem asymmetrischen dreiwertigen Stickstoffatom. VI. Mitteilung über die Alkaloide des Granatapfelbaumes". Berichte der deutschen chemischen Gesellschaft (A and B Series) 52 (5): 964. doi:10.1002/cber.19190520513.
- ^ Heß, Kurt; Weltzien, Wilhelm (1920). "Über die Fähigkeit der Pflanze, optische Antipoden aufzubauen". Berichte der deutschen chemischen Gesellschaft (A and B Series) 53 (2): 119. doi:10.1002/cber.19200530202.
- ^ Hess, Kurt; Grau, Reinhold (1925). "Neue Umwandlungen von Conhydrin und Methylisopelletierin. (V. Mitteilung zur Frage des asymmetrischen dreiwertigen Stickstoffatoms)". Justus Liebig's Annalen der Chemie 441: 101. doi:10.1002/jlac.19254410106.
- ^ Meisenheimer, Jokob; Mahler, Emil (1928). "Über das Methyl-isopelletierin. (VIII. Mitteilung zur Stereochemie des gesättigten dreiwertigen Stickstoffatoms)". Justus Liebig's Annalen der Chemie 462: 301. doi:10.1002/jlac.19284620119.
- ^ Engler, C.; Bauer, F. W. (1891). "Ueber das α-Aethylpyridylketon und dessen Ueberführung in Pseudoconhydrin". Berichte der deutschen chemischen Gesellschaft 24 (2): 2530. doi:10.1002/cber.18910240244.
- ^ Engler, C.; Bauer, F. W. (1894). "Die Reductionsproducte des α-Methylpyridylketons und die Nichtidentität des α-Aethyl-Piperylalkins mit dem activen Pseudoconhydrin". Berichte der deutschen chemischen Gesellschaft 27 (2): 1775. doi:10.1002/cber.189402702121.
Categories:- Piperidines
- Alkaloids
- Neurotoxins
Wikimedia Foundation. 2010.