- Cobalt tetracarbonyl hydride
-
Cobalt tetracarbonyl hydride  Other namescobalt hydrocarbonyl
Other namescobalt hydrocarbonyl
tetracarbonylhydridocobalt, TetracarbonylhydrocobaltIdentifiers CAS number 16842-03-8  =
=PubChem 61848 Properties Molecular formula C4HCoO4 Molar mass 171.98 g/mol Appearance Light yellow liquid Melting point -33 °C, 240 K, -27 °F
Boiling point 47 °C, 320 K, 117 °F
Solubility in water Partially Solubility soluble in hexane, toluene, ethanol Acidity (pKa) 8.5  tetracarbonyl hydride (verify) (what is:
tetracarbonyl hydride (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Cobalt tetracarbonyl hydride is the organometallic compound with the formula HCo(CO)4. It is a yellow liquid that forms a colorless vapor and has an intolerable odor.[1] Its main use is as a catalyst in hydroformylation.
Contents
Structure and properties
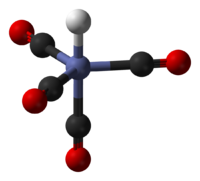
HCo(CO)4 is a trigonal bipyrimidal molecule. The hydride ligand occupies one of the axial positions, thus the symmetry of the molecule is C3v.[2] The Co-CO and Co-H bond distances were determined by gas-phase electron diffraction to be 1.764 and 1.556 Å, respectively.[3]. The oxidation state of cobalt in this compound is -1.
Like some other metal carbonyl hydrides, HCo(CO)4 is acidic, with a pKa of 8.5.[4] HCo(CO)4 melts at -33 °C and above that temperature decomposes to Co2(CO)8 and H2.[1] It undergoes substitution by tertiary phosphines. For example, triphenylphosphine gives HCo(CO)3PPh3 and HCo(CO)2(PPh3)2. These derivatives are more stable than HCo(CO)4 and are used industrially.[5] These derivatives are generally less acidic than HCo(CO)4.[4]
Preparation
Tetracarbonylhydrocobalt was first described by Hieber in the early 1930s.[6] It was the second transition metal hydride to be discovered, after H2Fe(CO)4. It is prepared by reducing Co2(CO)8 with sodium amalgam or a similar reducing agent followed by acidification.[2]
- Co2(CO)8 + 2 Na → 2 NaCo(CO)4
- NaCo(CO)4 + H+ → HCo(CO)4 + Na+
Since HCo(CO)4 decomposes so readily, it is usually generated in situ by hydrogenation of Co2(CO)8.[5]
The thermodynamic parameters for the equilibrium reaction were determined by infrared spectroscopy to be ΔH = 4.054 kcal mol−1, ΔS = -3.067 cal mol−1 K−1.[5]
Applications
Tetracarbonylhydridocobalt was the first transition metal hydride to be used in industry.[7] In 1940 it was discovered that it catalyzed the conversion of alkenes, CO, and H2 to aldehydes, a process known as hydroformylation (Oxo Reaction). Although it has since been largely superseded by rhodium-based catalysts, the world output of C3-C18 aldehydes produced by tetracarbonylhydrocobalt catalysis is about 100,000 tons/year, roughly 2% of the total.[7]
References
- ^ a b Kerr, W. J. (2001). "Sodium Tetracarbonylcobaltate". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rs105.
- ^ a b Donaldson, J. D.; Beyersmann, D. (2005). "Cobalt and Cobalt Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH. doi:10.1002/14356007.a07_281.pub2.
- ^ McNeill, E. A.; Scholer, F. R. (1977). "Molecular structure of the gaseous metal carbonyl hydrides of manganese, iron, and cobalt". Journal of the American Chemical Society 99: 6243. doi:10.1021/ja00461a011.
- ^ a b Moore, E. J.; Sullivan, J. M.; Norton, J. R. (1986). "Kinetic and thermodynamic acidity of hydrido transition-metal complexes. 3. Thermodynamic acidity of common mononuclear carbonyl hydrides". Journal of the American Chemical Society 108: 2257. doi:10.1021/ja00269a022.
- ^ a b c M. Pfeffer, M. Grellier "Cobalt Organometallics" in Comprehensive Organometallic Chemistry III, 2007, Elsevier.doi:10.1016/B0-08-045047-4/00096-0
- ^ Hieber, W.; Mühlbauer, F.; Ehmann, E. A. (1932). "Derivate des Kobalt- und Nickelcarbonyls (XVI. Mitteil. über Metallcarbonyle)". Berichte der deutschen chemischen Gesellschaft (A and B Series) 65: 1090. doi:10.1002/cber.19320650709.
- ^ a b Rittmeyer, P.; Wietelmann, U. (2000). "Hydrides". Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH. doi:10.1002/14356007.a13_199.
Hydrogen compounds H3AsO3 · H3AsO4 · HAt · HSO3F · HBF4 · HBr · HBrO · HBrO2 · HBrO3 · HBrO4 · HCl · HClO · HClO2 · HClO3 · HClO4 · HCN · HCNO · H2CrO4/H2Cr2O7 · H2CO3 · H2CS3 · HF · HFO · HI · HIO · HNC · HNCO · HNO · HNO3 · H2N2O2 · HNO5S · H3NSO3 · H2O · H2O2 · H2O3 · H3PO2 · H3PO3 · H3PO4 · H4P2O7 · H5P3O10 · H2PtCl6 · H2S · H2Se · H2SeO3 · H2SeO4 · H4SiO4 · H2SiF6 · H2SO3 · H2SO4 · H2SO5 · H2S2O3 · H2S2O6 · H2S2O7 · H2S2O8 · CF3SO3H · H2Te · H2TeO3 · H6TeO6 · H4TiO4 · H2Po · H3VO4 · HCo(CO)4
Categories:- Organocobalt compounds
- Metal hydrides
- Carbonyl complexes
Wikimedia Foundation. 2010.