- Palladium(II) chloride
-
Palladium(II) chloride 
Identifiers CAS number 7647-10-1 
EC number 231-596-2 RTECS number RT3500000 Properties Molecular formula PdCl2 Molar mass 177.33 g/mol Appearance dark red solid
hygroscopicDensity 4.0 g/cm3 Melting point 679 °C (decomp.)
Solubility in water soluble in trace amounts, better solubility in cold water Solubility soluble in organic solvents
dissolves rapidly in HClStructure Crystal structure rhombohedral Coordination
geometrysquare planar Hazards EU Index Not listed Flash point Non-flammable Related compounds Other anions Palladium(II) fluoride
Palladium(II) bromide
Palladium(II) iodideOther cations Nickel(II) chloride
Platinum(II) chloride
Platinum(II,IV) chloride
Platinum(IV) chloride chloride (verify) (what is:
chloride (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Palladium(II) chloride, also known as palladium dichloride, are the chemical compounds with the formula PdCl2. PdCl2 is a common starting material in palladium chemistry – palladium-based catalysts are of particular value in organic synthesis. It is prepared by chlorination of palladium.
Contents
Structure
Two forms of PdCl2 are known. In both forms, the palladium centres adopt the square-planar coordination geometry that is characteristic of Pd(II). Furthermore, in both forms, the Pd(II) centres are linked by μ2-chloride bridges. The α-form of PdCl2 is a polymer, consisting of "infinite" slabs or chains. The β-form of PdCl2 is molecular, consisting of an octahedral cluster of six Pd atoms. Each of the twelve edges of this octahedron is spanned by Cl−. PtCl2 adopts similar structures, whereas NiCl2 adopts the CdCl2 motif, featuring hexacoordinated Ni(II).[1]
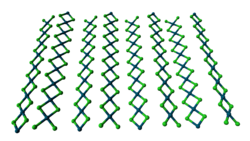
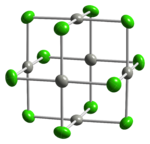
ball-and-stick model of the
crystal structure of α-PdCl2thermal ellipsoid model of the Pd6Cl12 molecule
found in the crystal structure of β-PdCl2Preparation
Palladium(II) chloride is prepared by dissolving palladium metal in aqua regia or hydrochloric acid in the presence of chlorine. Alternatively, it may be prepared by heating palladium sponge with chlorine gas at 500°C.
Reactions
Palladium(II) chloride is a common starting point in the synthesis of other palladium compounds. It is not particularly soluble in water or non-coordinating solvents, so the first step in its utilization is often the preparation of labile but soluble Lewis base adducts, such as those derived from acetonitrile or benzonitrile.[2] The acetonitrile complex is prepared by treating PdCl2 in refluxing acetonitrile:
- PdCl2 + 2 MeCN → PdCl2(MeCN)2
Although occasionally recommended, inert-gas techniques are not necessary if the complex is to be used in situ. As an example, bis(triphenylphosphine)palladium(II) dichloride may be prepared from palladium(II) chloride by reacting it with triphenylphosphine in benzonitrile:[3]
- PdCl2 + 2 PPh3 → PdCl2(PPh3)2
Further reduction in the presence of more triphenylphosphine gives tetrakis(triphenylphosphine)palladium(0); the second reaction may be carried out without purifying the intermediate dichloride:[4]
- PdCl2(PPh3)2 + 2 PPh3 + 2.5 N2H4 → Pd(PPh3)4 + 0.5 N2 + 2 N2H5+Cl−
Alternatively, palladium(II) chloride may be solubilized in the form of the tetrachloropalladate anion, e.g. sodium tetrachloropalladate, by reacting with the appropriate alkali metal chloride in water:[5] Palladium(II) chloride is insoluble in water, whereas the product dissolves:
- PdCl2 + 2 MCl → M2PdCl4
This compound may also further react with phosphines to give phosphine complexes of palladium.[5]
Palladium chloride may also be used to give heterogeneous palladium catalysts: palladium on barium sulfate, palladium on carbon, and palladium chloride on carbon.[6]
Uses
Even when dry, palladium(II) chloride is able to rapidly stain stainless steel. Thus, palladium(II) chloride solutions are sometimes used to test for the corrosion-resistance of stainless steel.[7]
Palladium(II) chloride is sometimes used in carbon monoxide detectors. Carbon monoxide reduces palladium(II) chloride to palladium:
- PdCl2 + CO + H2O → Pd + CO2 + 2HCl
Residual PdCl2 is converted to red PdI2, the concentration of which may be determined colorimetrically:[8]
- PdCl2 + 2 KI → PdI2 + 2 KCl
References
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ^ Gordon K. Anderson, Minren Lin (1990). "Bis(Benzonitrile)Dichloro Complexes of Palladium and Platinum". Inorg. Synth. 28: 60–63. doi:10.1002/9780470132593.ch13.
- ^ Norio Miyaura and Akira Suzuki (1993), "Palladium-catalyzed reaction of 1-alkenylboronates with vinylic halides: (1Z,3E)-1-Phenyl-1,3-octadiene", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv8p0532; Coll. Vol. 8: 532
- ^ D. R. Coulson; Satek, L. C.; Grim, S. O. (1972). "23. Tetrakis(triphenylphosphine)palladium(0)". Inorg. Synth. 13: 121. doi:10.1002/9780470132449.ch23.
- ^ a b Daniele Choueiry and Ei-ichi Negishi (2002). "II.2.3 Pd(0) and Pd(II) Complexes Containing Phosphorus and Other Group 15 Atom Ligands". In Ei-ichi Negishi (Google Books excerpt). Handbook of Organopalladium Chemistry for Organic Synthesis. John Wiley & Sons, Inc.. ISBN 0-471-31506-0. http://books.google.com/?id=mTMA2hExAaIC&pg=PA47.
- ^ Ralph Mozingo (1955), "Palladium Catalysts", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv3p0685; Coll. Vol. 3: 685
- ^ For example, http://www.marinecare.nl/assets/Uploads/Downloads/Leaflet-Passivation-Test-Kit.pdf
- ^ T. H. Allen, W. S. Root (1955). "Colorimetric Determination of Carbon Monoxide in Air by an improved Palladium Chloride Method". J. Biol. Chem. 216 (1): 309–317. PMID 13252030. http://www.jbc.org/content/216/1/309.
Palladium compounds Pd(OAc)2 · Pd(C5H7O2)2 · PdBr2 · PdCl2 · Pd(CN)n · PdF2 · PdF3 · PdF4 · PdI2 · Pd(NO3)2 · PdO · C6H10Cl2Pd2 · C34H28Cl2FeP2Pd · C36H30Cl2P2Pd · C72H60P4Pd
Categories:- Palladium compounds
- Chlorides
- Metal halides
Wikimedia Foundation. 2010.

