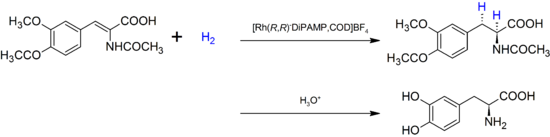- Chiral ligand
-
In chemistry a chiral ligand is a specially adapted ligand used for asymmetric synthesis. This ligand is an enantiopure organic compound which combines with a metal center by chelation to form an asymmetric catalyst. This catalyst engages in a chemical reaction and transfers its chirality to the reaction product which as a result also becomes chiral. In an ideal reaction one equivalent of catalyst can turn over many more equivalents of reactant which enables the synthesis of a large amount of a chiral compound from achiral precursors with the aid of a very small (often expensive) chiral ligand.
Contents
First discovery
The first such ligand, the diphosphine DiPAMP was developed in 1968 by William S. Knowles of Monsanto Company, who won the 2001 Nobel Prize in Chemistry,[1] and ultimately used in the industrial production of L-DOPA.
Privileged ligands
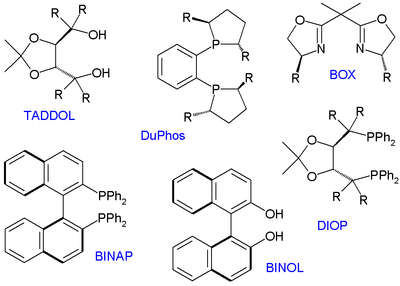
Many thousands of chiral ligands have been prepared and tested since then but only several compound classes have been found to have a general scope. These ligands are therefore called privileged ligands.[2][3] Important members depicted below are BINOL, BINAP, TADDOL, DIOP, BOX and DuPhos (a phosphine ligand), all available as enantiomeric pairs.
Other members are Salen , cinchona alkaloids and phosphoramidites. Many of these ligands possess C2 symmetry which limits the number of possible reaction pathways and thereby increasing enantioselectivity.
Chiral fence
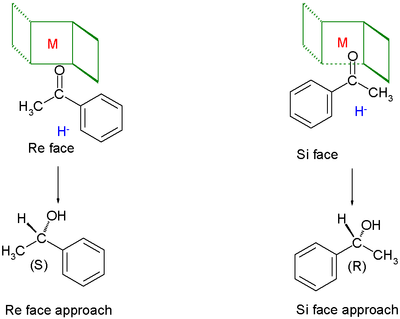
Chiral ligands work asymmetric induction somewhere along the reaction coordinate. The image depicted on the right gives a general idea how a chiral ligand may induce an enantioselective reaction. The ligand (in green) has C2 symmetry with its nitrogen, oxygen or phosphorus atoms hugging a central metal atom (in red). In this particular ligand the right side is sticking out and its left side points away. The substrate in this reduction is acetophenone and the reagent (in blue) a hydride ion. In absence of the metal and the ligand the re face approach of the hydride ion gives the (S)-enantiomer and the si face approach the (R)-enantiomer in equal amounts (a racemic mixture like expected). The ligand/metal presence changes all that. The carbonyl group will coordinate with the metal and due to the steric bulk of the phenyl group it will only be able to do so with its si face exposed to the hydride ion with in the ideal situation exclusive formation of the (R) enantiomer. The re face will simple hit the chiral fence.[4] Note that when the ligand is replaced by its mirror image the other enantiomer will form and that a racemic mixture of ligand will once again yield a racemic product. Also note that if the steric bulk of both carbonyl substituents is very similar the strategy will fail.
Chiral counterions
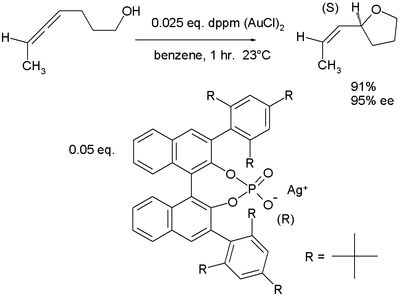
In a novel concept, so-called chiral ions team up with traditional cationic catalysts in asymmetric synthesis as demonstrated in this allene hydroxyalkoxylation in which the active catalyst is a salt of gold(I) and a phosphate of a chiral binaphthol:[5][6]
References
- ^ Nobel prize 2001 www.nobelprize.org Link
- ^ Design of chiral ligands for asymmetric catalysis: From C2-symmetric P,P- and N,N-ligands to sterically and electronically nonsymmetrical P,N-ligands Andreas Pfaltz and William J. Drury III PNAS, April 20, 2004 vol. 101 no. 16 5723-5726 doi:10.1073/pnas.0307152101
- ^ Privileged Chiral Catalysts Tehshik P. Yoon, Eric N. Jacobsen Science 14 March 2003: Vol. 299. no. 5613, pp. 1691 - 1693 doi:10.1126/science.1083622 PMID 12637734
- ^ Chiral and C2-symmetrical bis(oxazolinylpyridine)rhodium(III) complexes: effective catalysts for asymmetric hydrosilylation of ketones Hisao Nishiyama, Hisao Sakaguchi, Takashi Nakamura, Mihoko Horihata, Manabu Kondo, and Kenji Itoh Organometallics; 1989; 8(3) pp 846 - 848; doi: 10.1021/om00105a047
- ^ A Powerful Chiral Counterion Strategy for Asymmetric Transition Metal Catalysis Gregory L. Hamilton, Eun Joo Kang, Miriam Mba, F. Dean Toste. Science 317, 496 (2007) doi: 10.1126/science.1145229
- ^ Starting catalyst: 1,2-bis(diphenylphosphino)ethane (dppm) gold(I) chloride complex
Concepts in asymmetric synthesis Chirality types Chirality · Stereocenter · Planar chirality · Chiral ligand · Axial chirality · Supramolecular chirality · Inherent chiralityChiral molecules Stereoisomer · Enantiomer · Diastereomer · Meso compound · Enantiomeric excess · Diastereomeric excess ·Analysis Optical rotation · Chiral derivatizing agents · NMR spectroscopy of stereoisomers · Ultraviolet-visible spectroscopy of stereoisomersChiral resolution Recrystallization · Kinetic resolution · Chiral column chromatography · Diastereomeric recrystallizationReactions Categories:
Wikimedia Foundation. 2010.