- Claisen rearrangement
-
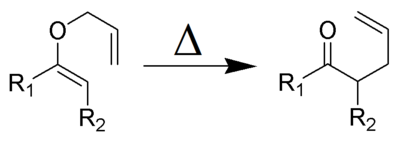
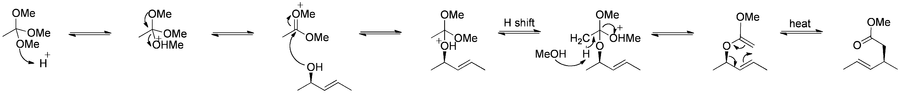
The Claisen rearrangement (not to be confused with the Claisen condensation) is a powerful carbon-carbon bond-forming chemical reaction discovered by Rainer Ludwig Claisen. The heating of an allyl vinyl ether will initiate a [3,3]-sigmatropic rearrangement to give a γ,δ-unsaturated carbonyl.
Discovered in 1912, the Claisen rearrangement is the first recorded example of a [3,3]-sigmatropic rearrangement.[1][2][3]
Many reviews have been written.[4][5][6][7]
Contents
Mechanism
The Claisen rearrangement (and its variants) are exothermic (about 84 kJ/mol), concerted pericyclic reactions which according to the Woodward-Hoffmann rules show a suprafacial reaction pathway.
There are substantial solvent effects in the Claisen reactions. More polar solvents tend to accelerate the reaction to a greater extent. Hydrogen-bonding solvents gave the highest rate constants. For example, ethanol/water solvent mixtures give rate constants 10-fold higher than sulfolane.[1][2]
Trivalent organoaluminium reagents, such as trimethylaluminium, have been shown to accelerate this reaction.[8][9]
Variations
Aromatic Claisen rearrangement
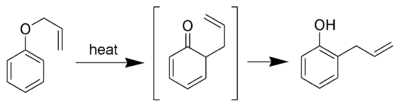
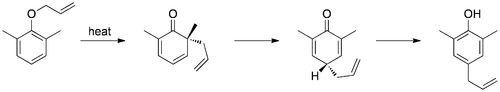
The aromatic variation of the Claisen rearrangement is the [3,3]-sigmatropic rearrangement of an allyl phenyl ether to an intermediate which quickly tautomerizes to an ortho-substituted phenol.
If ortho position is substituted then reaction goes to para position with retention in configration.[10]
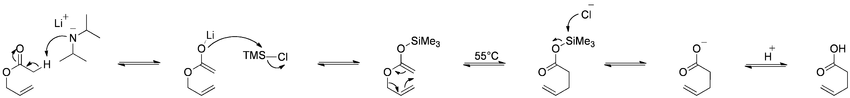
Bellus-Claisen rearrangement
The Bellus-Claisen rearrangement is the reaction of allylic ethers, amines, and thioethers with ketenes to give γ,δ-unsaturated esters, amides, and thioesters.[11][12][13]

Eschenmoser-Claisen rearrangement
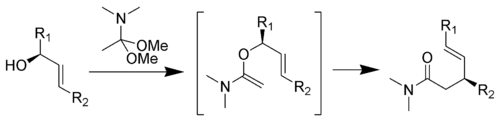
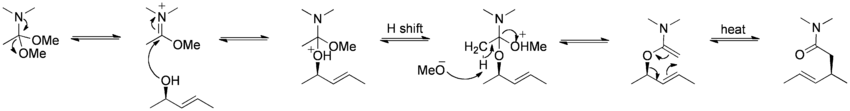
The Eschenmoser-Claisen rearrangement proceeds from an allylic alcohol to a γ,δ-unsaturated amide, and was developed by Albert Eschenmoser in 1964.[14][15]
Mechanism:[10]
Ireland-Claisen rearrangement
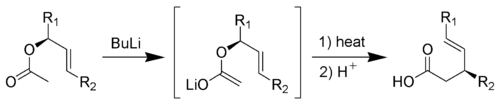
Main article: Ireland-Claisen rearrangementThe Ireland-Claisen rearrangement is the reaction of an allylic acetate with strong base (such as Lithium diisopropylamide) to give a γ,δ-unsaturated carboxylic acid.[16][17][18]
Mechanism:[10]
Johnson-Claisen rearrangement
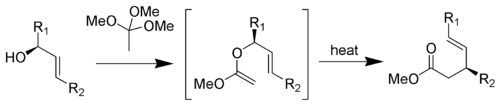
The Johnson-Claisen rearrangement is the reaction of an allylic alcohol with trimethyl orthoacetate to give a γ,δ-unsaturated ester.[19]
Mechanism:[10]
Hetero-Claisens
Aza-Claisen
An iminium can serve as one of the pi-bonded moieties in the rearrangement.[20]
Chromium Oxidation
Chromium can oxidize allylic alcohols to alpha-beta unsaturated ketones on the opposite side of the unsaturated bond from the alcohol. This is via a concerted hetero-claisen reaction, although there are mechanistic differences since the chromium atom has access to d- shell orbitals which allow the reaction under a less constrained set of geometries.[21][22]

Chen-Mapp Reaction
The Chen-Mapp reaction also known as the [3,3]-Phosphorimidate Rearrangement or Staudinger-Claisen Reaction installs a phosphite in the place of an alcohol and takes advantage of the Staudinger Ligation to convert this to an imine. The subsequent claisen is driven by the fact that a P=O double bond is more energetically favorable than a P=N double bond.[23]

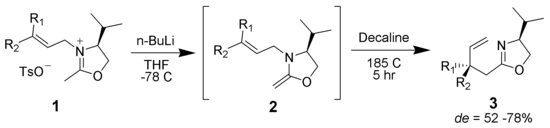
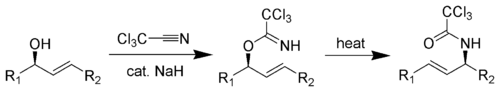
Overman rearrangement
Main article: Overman rearrangementThe Overman rearrangement (named after Larry Overman) is a Claisen rearrangement of allylic trichloroacetimidates to allylic trichloroacetamides.[24][25][26]
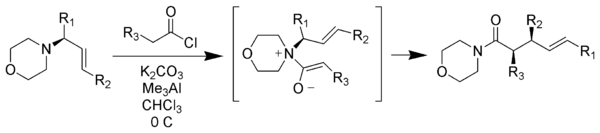
Zwitterionic Claisen rearrangement
Unlike typical Claisen rearrangements which require heating, zwitterionic Claisen rearrangements take place at or below room temperature. The acyl ammonium ions are highly selective for Z-enolates under mild conditions.[27][28]
Claisen rearrangement in nature
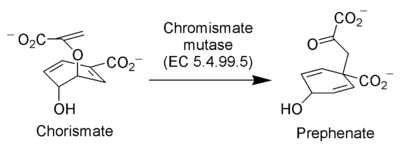
The enzyme Chorismate mutase (EC 5.4.99.5) catalyzes the Claisen rearrangement of chorismate ion to prephenate ion, a key intermediate in the shikimic acid pathway (the biosynthetic pathway towards the synthesis of phenylalanine and tyrosine).[29]
References
- ^ Claisen, L.; Ber. 1912, 45, 3157.
- ^ Claisen, L.; Tietze, E.; Ber. 1925, 58, 275.
- ^ Claisen, L.; Tietze, E.; Ber. 1926, 59, 2344.
- ^ Hiersemann, M.; Nubbemeyer, U. (2007) The Claisen Rearrangement. Wiley-VCH. ISBN 3-527-30825-3
- ^ Rhoads, S. J.; Raulins, N. R.; Org. React. 1975, 22, 1-252. (Review)
- ^ Ziegler, F. E.; Chem. Rev. 1988, 88, 1423-1452. (Review)
- ^ Wipf, P.; Comp. Org. Syn. 1991, 5, 827-873.
- ^ Goering, H. L.; Jacobson, R. R.; J. Am. Chem. Soc. 1958, 80, 3277.
- ^ White, W. N.; Wolfarth, E. F.; J. Org. Chem. 1970, 35, 2196.
- ^ Kurti, Laszlo and Barbara Czako. Strategic Applications of Named Reactions in Organic Synthesis: Background and Detailed Mechanisms. Amsterdam. Elsevier Academic Press: 2005.
- ^ Malherbe, R.; Bellus, D.; Helv. Chim. Acta 1978, 61, 3096-3099.
- ^ Malherbe, R.; Rist, G.; Bellus, D.; J. Org. Chem. 1983, 48, 860-869.
- ^ Gonda, J.; Angew. Chem. Int. Ed. 2004, 43, 3516-3524.
- ^ Wick, A. E.; Felix, D.; Steen, K.; Eschenmoser, A.; Helv. Chim. Acta 1964, 47, 2425-2429.
- ^ Wick, A. E.; Felix, D.; Gschwend-Steen, K.; Eschenmoser, A.; Helv. Chim. Acta 1969, 52, 1030-1042.
- ^ Ireland, R. E.; Mueller, R. H. (1972). "Claisen rearrangement of allyl esters". Journal of the American Chemical Society 94 (16): 5897. doi:10.1021/ja00771a062.
- ^ Ireland, R. E.; Willard, A. K.; Tetrahedron Lett. 1975, 16, 3975-3978.
- ^ Ireland, R. E.; Mueller, R. H.; Willard, A. K. (1976). "The ester enolate Claisen rearrangement. Stereochemical control through stereoselective enolate formation". Journal of the American Chemical Society 98 (10): 2868. doi:10.1021/ja00426a033.
- ^ Johnson, W. S. et al.; J. Am. Chem. Soc. 1970, 92, 741.
- ^ Kurth, M. J.; Decker, O. H. W.; J. Org. Chem. 1985, 50, 5769-5775.
- ^ Dauben, W. G.; Michno, D. M. J. Org. Chem., 1977, 42, 682.
- ^ Organic Syntheses, Vol. 82, p.108 (2005). (Article)
- ^ Chen, B.; Mapp, A. (2005). "Thermal and catalyzed 3,3-phosphorimidate rearrangements". Journal of the American Chemical Society 127 (18): 6712–6718. doi:10.1021/ja050759g. PMID 15869293.
- ^ Overman, L. E. (1974). "Thermal and mercuric ion catalyzed [3,3]-sigmatropic rearrangement of allylic trichloroacetimidates. 1,3 Transposition of alcohol and amine functions". Journal of the American Chemical Society 96 (2): 597–599. doi:10.1021/ja00809a054.
- ^ Overman, L. E. (1976). "A general method for the synthesis of amines by the rearrangement of allylic trichloroacetimidates. 1,3 Transposition of alcohol and amine functions". Journal of the American Chemical Society 98 (10): 2901–2910. doi:10.1021/ja00426a038.
- ^ Organic Syntheses, Coll. Vol. 6, p.507; Vol. 58, p.4 (Article)
- ^ Yu, C.-M.; Choi, H.-S.; Lee, J.; Jung, W.-H.; Kim, H.-J. J. Chem. Soc., Perkin Trans. 1 1996, 115-116.
- ^ Nubbemeyer, U. J. Org. Chem. 1995, 60, 3773-3780.
- ^ Ganem, B. Angew. Chem. Int. Ed. Engl. 1996, 35, 936-945.
See also
Categories:- Rearrangement reactions
- Name reactions
Wikimedia Foundation. 2010.