- Chiral pool synthesis
-
Chiral pool synthesis is a strategy that aims to improve the efficiency of chiral synthesis. It starts the organic synthesis of a complex enantiopure chemical compound from a stock of readily available enantiopure substances. Common chiral starting materials include monosaccharides and amino acids. The built-in chirality is then preserved in the remainder of the reaction sequence.
This strategy is especially helpful if the desired molecule bears a great resemblance to cheap enantiopure natural products. Otherwise, a long, tortuous synthesis involving many steps with attendant losses in yield may be required. At times, it may be difficult to find a suitable enantiopure starting material; other techniques may prove more fruitful.
General methods used in chiral pool synthesis are the use of protecting groups, and functional group interconversion (FGI).
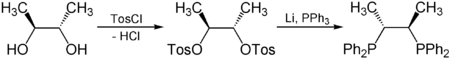
Examples
Chiral pool synthesis is used to build a part of the epothilone molecule (an alternative to Paclitaxel), from readily available enantiopure (–)-pantolactone.[1] Enantiopure tartaric acid is also used to synthesize chiraphos:[2]
Enantiomeric scaffolding
Enantiomeric scaffolding is a related concept whereby a conceptually simple but synthetic core molecule of high enantiopurity with many functional groups is synthesized from which a diverse family of molecules can be constructed [3].
References
- ^ Ulrich Klar, et al. (2005). "Efficient Chiral Pool Synthesis of the C1-C6 Fragment of Epothilones". Synthesis 2005 (2): 301–305. doi:10.1055/s-2004-834936.
- ^ M. D. Fryzuk, B. Bosnich (1977). "Asymmetric synthesis. Production of optically active amino acids by catalytic hydrogenation". J. Am. Chem. Soc. 99 (19): 6262–6267. doi:10.1021/ja00461a014. PMID 893889.
- ^ Practical, Scalable, High-Throughput Approaches to 3-Pyranyl and 3-Pyridinyl Organometallic Enantiomeric Scaffolds Using the Achmatowicz Reaction Thomas C. Coombs, Maurice D. Lee, IV, Heilam Wong, Matthew Armstrong, Bo Cheng, Wenyong Chen, Alessandro F. Moretto, and Lanny S. Liebeskind J. Org. Chem. 73 (3), 882 -888, 2008. doi:10.1021/jo702006z
Concepts in asymmetric synthesis Chirality types Chirality · Stereocenter · Planar chirality · Chiral ligand · Axial chirality · Supramolecular chirality · Inherent chiralityChiral molecules Stereoisomer · Enantiomer · Diastereomer · Meso compound · Enantiomeric excess · Diastereomeric excess ·Analysis Optical rotation · Chiral derivatizing agents · NMR spectroscopy of stereoisomers · Ultraviolet-visible spectroscopy of stereoisomersChiral resolution Recrystallization · Kinetic resolution · Chiral column chromatography · Diastereomeric recrystallizationReactions Asymmetric induction · Chiral pool synthesis · Chiral auxiliaries · Asymmetric catalysis · Organocatalysis · BiocatalysisCategories:
Wikimedia Foundation. 2010.