- Desoxymethyltestosterone
-
Desoxymethyltestosterone 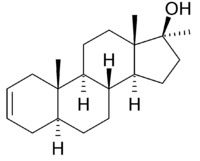
Systematic (IUPAC) name (5S,8R,9S,10S,13S,14S,17S)-10,13,17-trimethyl-1,4,5,6,7,8,9,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-17-ol Clinical data Pregnancy cat. X Legal status Schedule III (US) Routes Oral, IM, Transdermal Identifiers CAS number 3275-64-7 ATC code None PubChem CID 18651 ChemSpider 17611 
Synonyms Desoxymethyltestosterone; Madol; Pheraplex; 17α-Methyl-5α-androst-2-en-17β-ol Chemical data Formula C20H32O Mol. mass 288.46748 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Desoxymethyltestosterone (Madol, Pheraplex) is an anabolic steroid. It was one of the first "designer steroids" to be marketed as a performance-enhancing drug to athletes and bodybuilders. In animal studies it has been found to bind to the androgen receptor about as half as strongly as dihydrotestosterone, and caused side effects that are typical of 17α-alkylated steroids, such as liver damage when taken in higher dosages and left ventricular hypertrophy.[1]
Desoxymethyltestosterone is sometimes referred to as "DMT", though it is not the same compound as the hallucinogen dimethyltryptamine, also known as DMT.
Desoxymethyltestosterone was invented in 1961 by Max Huffman who obtained a patent on the compound. It was never brought to market as a commercial drug. It was rediscovered by chemist Patrick Arnold in 2005. Arnold produced desoxymethyltestosterone and supplied it to Victor Conte of Bay Area Laboratory Co-operative (BALCO), an American nutritional supplement company and steroid supplier.[2]
Desoxymethyltestosterone is unusual in that it is structurally a 2-ene compound, lacking the 3-keto group present on nearly all commercial anabolic steroids. This does not mean it is a weak compound, and clinical research has determined that it is a fairly potent oral agent.[1] Rat studies indicate desoxymethyltestosterone has an anabolic effect 160% that of testosterone while being only 60% as androgenic giving it a Q ratio of 6.5:1.[3] Because of this favorable ratio, experiments in orchiectomised rats have demonstrated that treatment with desoxymethyltestosterone resulted only in a stimulation of the weight of the levator ani muscle; the prostate and seminal vesicle weights remained unaffected leading the authors of one study to characterize DMT as a powerful anabolic steroid with SARM (selective androgenic receptor modulator) attributes and some indication of toxicity. [1]
DMT became a controlled substance in the US on January 4, 2010, and is classified as a Schedule III anabolic steroid under the United States Controlled Substances Act along with boldione and dienedione.[4][5][6][7] The substance had come under scrutiny after it was found to be present in several over-the-counter bodybuilding supplements[8].
References
- ^ a b c Diel P, Friedel A, Geyer H, Kamber M, Laudenbach-Leschowsky U, Schänzer W, Thevis M, Vollmer G, Zierau O (2007). "Characterisation of the pharmacological profile of desoxymethyltestosterone (Madol), a steroid misused for doping". Toxicology Letters 169 (1): 64–71. doi:10.1016/j.toxlet.2006.12.004. PMID 17254722.
- ^ "Chemist Who Created "The Clear" Sentenced" (Press release). United States Attorney for the Northern District of California. 2006-08-04. http://www.usdoj.gov/usao/can/press/2006/2006_08_04_arnold_sentencing%20press.htm. Retrieved 2007-10-08.
- ^ Edwards J.A. and Bowers A. (1961). Chemistry and Industry: 1962–63.
- ^ Rules - 2008 - Classification of Three Steroids as Schedule III Anabolic Steroids Under the Controlled Substances Act
- ^ FR Doc E8-8842
- ^ DEA proposes listing three steroids under Schedule III - Since three steroids have a high potential for drug abuse and trafficking, the Drug Enforcement Administration wants to classify them as Schedu
- ^ Classification of Three Steroids as Schedule III Anabolic Steroids Under the Controlled Substances Act
- ^ http://www.ncbi.nlm.nih.gov/pubmed/20355167
Categories:- Anabolic steroids
Wikimedia Foundation. 2010.
