- Embryo transfer
-
Embryo transfer Intervention 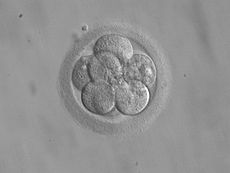
8-cell embryo for transfer 3 days after fertilizationMeSH D004624 Embryo transfer refers to a step in the process of assisted reproduction in which embryos are placed into the uterus of a female with the intent to establish a pregnancy. This technique (which is often used in connection with in vitro fertilization (IVF)), may be used in humans or in animals, in which situations the goals may vary.
Contents
Fresh versus frozen
Embryos can be either “fresh” from fertilized egg cells of the same menstrual cycle, or “frozen”, that is they have been generated in a preceding cycle and undergone embryo cryopreservation, and are thawed just prior to the transfer. The outcome from using cryopreserved embryos has uniformly been positive with no increase in birth defects or development abnormalities,[1] also between fresh versus frozen eggs used for intracytoplasmic sperm injection (ICSI).[2] Children born from vitrified blastocysts have significantly higher birthweight than those born from non-frozen blastocysts.[3]
Uterine preparation
In the human, the uterine lining (endometrium) needs to be appropriately prepared so that the embryo(s) can implant. In a natural or stimulated cycle, the embryo transfer takes place in the luteal phase at a time where the lining is appropriately undeveloped in relation to the status of the present Luteinizing Hormone. In a cycle where a "frozen" embryo is transferred, the recipient woman could be given first estrogen preparations (about 2 weeks), then a combination of oestrogen and progesterone so that the lining becomes receptive for the embryo. The time of receptivity is the implantation window.
Limited evidence also supports removal of cervical mucus before transfer.[4]
Timing
In stimulated cycles in human IVF, embryos are typically transferred 3 days after fertilization and may then be at the eight-cell stage, or they are transferred 2 to 3 days later when they have reached the blastocyst stage. Embryos who reach the day 3 cell stage can be tested for chromosal or specific genetic defects prior to possible transfer by preimplantation genetic diagnosis (PGD).
Monozygotic twinning is not increased after blastocyst transfer compared with cleavage-stage embryo transfer.[5]
Procedure
The embryo transfer procedure starts by placing a speculum in the vagina to visualize the cervix, which is cleansed with saline solution or culture media. A soft[4] transfer catheter is loaded with the embryos and handed to the clinician after confirmation of the patient’s identity. The catheter is inserted through the cervical canal and advanced into the uterine cavity.
There is good and consistent evidence of benefit in ultrasound guidance,[4] that is, making an abdominal ultrasound to ensure correct placement, which is 1–2 cm from the uterine fundus. Anesthesia is generally not required. Single embryo transfers in particular require accuracy and precision in placement within the uterine cavity. The optimal target for embryo placement, known as the maximal implantation potential (MIP) point, is identified using 3D/4D ultrasound.[6] However, there is limited evidence that supports deposition of embryos in the midportion of the uterus.[4]
After insertion of the catheter, the contents are expelled and the embryos are deposited. Limited evidence supports making trial transfers before performing the procedure with embryos.[4] After expulsion, the duration that the catheter remains inside the uterus has no effect on pregnancy rates.[7] Limited evidence suggests avoiding negative pressure from the catheter after expulsion.[4] After withdrawal, the catheter is handed to the embryologist, who inspects it for retained embryos.
In the process of zygote intrafallopian transfer (ZIFT), eggs are removed from the woman, fertilised, and then placed in the woman's fallopian tubes rather than the uterus.
Embryo number
A major issue is how many embryos should be transferred. Placement of multiple embryos carries the risk of multiple pregnancy. In the past, physicians have often placed too many embryos in the hope to establish a pregnancy. However, the rise in multiple pregnancies has led to a reassessment of this approach. Professional societies and in many countries, the legislature, have issued guidelines or laws to curtail a practice of placing too many embryos in an attempt to reduce multiple pregnancies.
e-SET
The technique of selecting only one embryo to transfer to the woman is called elective-Single Embryo Transfer (e-SET) or, when embryos are at the blastocyst stage, it can also be called elective single blastocyst transfer (eSBT).[8] It lowers the risk of multiple pregnancies, compared with e.g. Double Embryo Transfer (DET) or double blastocyst transfer (2BT), with a twinning rate of approximately 3.5% in sET compared with approximately 38% in DET,[9] or 2% in eSBT compared with approximately 25% in 2BT.[8] At the same time, pregnancy rates is not significantly less with eSBT than with 2BT.[8] Furthermore, SET has better outcomes in terms of mean gestational age at delivery, mode of delivery, birthweight, and risk of neonatal intensive care unit necessity than DET.[9] e-SET of embryos at the cleavage stage reduces the likelihood of live birth by 38% and multiple birth by 94%.[10] Evidence from randomized, controlled trials suggests that increasing the number of e-SET attempts (fresh and/or frozen) results in a cumulative live birth rate similar to that of DET.[10]
The usage of single embryo transfer is highest in Sweden (69.4%), but as low as 2.8% in the USA. Access to public funding for ART, availability of good cryopreservation facilities and legislation appear to be the most important factors for regional usage of single embryo transfer.[11] Also, personal choice plays a significant role as many subfertile couples have a strong preference for twins.[11]
Follow-up
Patients usually start progesterone medication after egg (also called oocyte) retrieval. While daily intramuscular injections of progesterone-in-oil (PIO) have been the standard route of administration, PIO injections are not FDA-approved for use in pregnancy. A recent meta-analysis showed that the intravaginal route with an appropriate dose and dosing frequency is equivalent to daily intramuscular injections.[12] In addition, a recent case-matched study comparing vaginal progesterone with PIO injections showed that live birth rates were nearly identical with both methods.[13] A duration of progesterone administration of 11 days results in almost the same birth rates as longer durations.[14]
Patients are also given estrogen medication in some cases after the embryo transfer. Pregnancy testing is done typically two weeks after egg retrieval.
Third-party reproduction
It is not necessary that the embryo transfer be performed on the female who provided the eggs. Thus another female whose uterus is appropriately prepared can receive the embryo and become pregnant. Embryo transfer may be used where a woman who has eggs but no uterus and wants to have a biological baby; she would require the help of a gestational carrier or surrogate to carry the pregnancy. Also, a woman who has no eggs but a uterus may resort to egg donor IVF, in which case another woman would provide eggs for fertilization and the resulting embryos are placed into the uterus of the patient. Fertilization may be performed using the woman's partner's sperm or by using donor sperm. 'Spare' embryos which are created for another couple undergoing IVF treatment but which are then surplus to that couple's needs may also be transferred. Embryos may be specifically created by using eggs and sperm from donors and these can then be transferred into the uterus of another woman. A surrogate may carry a baby produced by embryo transfer for another couple, even though neither she nor the 'commissioning' couple is biologically related to the child. Third party reproduction is controversial and regulated in many countries. Persons entering gestational surrogacy arrangements must make sense of an entirely new type of relationship that does not fit any of the traditional scripts we use to categorize relations as kinship, friendship, romantic partnership or market relations.[15] Surrogates have the experience of carrying a baby that they conceptualize as not of their own kin, while intended mothers have the experience of waiting through nine months of pregnancy and transitioning to motherhood from outside of the pregnant body. This can lead to new conceptualizations of body and self.[15]
History
The first transfer of an embryo from one human to another resulting in pregnancy was reported in July 1983 and subsequently led to the announcement of the first human birth February 3, 1984.[16] This procedure was performed at the Harbor UCLA Medical Center [17] under the direction of Dr. John Buster and the University of California at Los Angeles School of Medicine.
In the procedure, an embryo that was just beginning to develop was transferred from one woman in whom it had been conceived by artificial insemination to another woman who gave birth to the infant 38 weeks later. The sperm used in the artificial insemination came from the husband of the woman who bore the baby.[18][19]
This scientific breakthrough established standards and became an agent of change for women suffering from the afflictions of infertility and for women who did not want to pass on genetic disorders to their children. Donor embryo transfer has given women a mechanism to become pregnant and give birth to a child that will contain their husband’s genetic makeup. Although donor embryo transfer as practiced today has evolved from the original non-surgical method, it now accounts for approximately 5% of in vitro fertilization recorded births.
Prior to this, thousands of women who were infertile, had adoption as the only path to parenthood. This set the stage to allow open and candid discussion of embryo donation and transfer. This breakthrough has given way to the donation of human embryos as a common practice similar to other donations such as blood and major organ donations. At the time of this announcement the event was captured by major news carriers and fueled healthy debate and discussion on this practice which impacted the future of reproductive medicine by creating a platform for further advancements in woman's health.
This work established the technical foundation and legal-ethical framework surrounding the clinical use of human oocyte and embryo donation, a mainstream clinical practice, which has evolved over the past 25 years.[18][19] Building upon this groundbreaking research and since the initial birth announcement in 1984, well over 47,000 live births resulting from donor embryo transfer have been and continue to be recorded by the Centers for Disease Control(CDC)[20] in the United States to infertile women, who otherwise would not have had children by any other existing method.[21][22]
Embryo transfer in animals
Embryo transfer techniques allow top quality female livestock to have a greater influence on the genetic advancement of a herd or flock in much the same way that artificial insemination has allowed greater use of superior sires.[23] ET also allows the continued use of animals such as competition mares to continue training and showing, while producing foals. The general epidemiological aspects of embryo transfer indicates that the transfer of embryos provides the opportunity to introduce genetic material into populations of livestock while greatly reducing the risk for transmission of infectious diseases. Recent developments in the sexing of embryos before transfer and implanting has great potential in the dairy and other livestock industries.[24]
Embryo transfer is also used in laboratory mice. For example, embryos of genetically modified strains that are difficult to breed or expensive to maintain may be stored frozen, and only thawed and implanted into a pseudopregnant dam when needed.
References
- ^ "Genetics & IVF Institute". Givf.com. Archived from the original on 2009-07-27. http://web.archive.org/web/*/http://givf.com/embryov.cfm. Retrieved 2009-07-27.
- ^ Wennerholm, U. -B.; Soderstrom-Anttila, V.; Bergh, C.; Aittomaki, K.; Hazekamp, J.; Nygren, K. -G.; Selbing, A.; Loft, A. (2009). "Children born after cryopreservation of embryos or oocytes: A systematic review of outcome data". Human Reproduction 24 (9): 2158–2172. doi:10.1093/humrep/dep125. PMID 19458318.
- ^ Wikland M, Hardarson T, Hillensjö T, et al. (May 2010). "Obstetric outcomes after transfer of vitrified blastocysts". Hum Reprod 25 (7): 1699–707. doi:10.1093/humrep/deq117. PMID 20472913.
- ^ a b c d e f Mains L, Van Voorhis BJ (April 2010). "Optimizing the technique of embryo transfer". Fertil Steril 94 (3): 785–90. doi:10.1016/j.fertnstert.2010.03.030. PMID 20409543.
- ^ Papanikolaou EG, Fatemi H, Venetis C, et al. (February 2009). "Monozygotic twinning is not increased after single blastocyst transfer compared with single cleavage-stage embryo transfer". Fertil. Steril. 93 (2): 592–7. doi:10.1016/j.fertnstert.2008.12.088. PMID 19243755.
- ^ Gergely RZ, DeUgarte CM, Danzer H, Surrey M, Hill D, DeCherney AH (2005). "Three dimensional/four dimensional ultrasound-guided embryo transfer using the maximal implantation potential point". Fertil. Steril. 84 (2): 500–3. doi:10.1016/j.fertnstert.2005.01.141. PMID 16084896..
- ^ Sroga JM, Montville CP, Aubuchon M, Williams DB, Thomas MA (April 2010). "Effect of delayed versus immediate embryo transfer catheter removal on pregnancy outcomes during fresh cycles". Fertil. Steril. 93 (6): 2088–90. doi:10.1016/j.fertnstert.2009.07.1664. PMID 20116786.
- ^ a b c Mullin CM, Fino ME, Talebian S, Krey LC, Licciardi F, Grifo JA (April 2010). "Comparison of pregnancy outcomes in elective single blastocyst transfer versus double blastocyst transfer stratified by age". Fertil. Steril. 93 (6): 1837–43. doi:10.1016/j.fertnstert.2008.12.137. PMID 19249756.
- ^ a b Fauque P, Jouannet P, Davy C, et al. (May 2009). "Cumulative results including obstetrical and neonatal outcome of fresh and frozen-thawed cycles in elective single versus double fresh embryo transfers". Fertil. Steril. 94 (3): 927–35. doi:10.1016/j.fertnstert.2009.03.105. PMID 19446806.
- ^ a b Gelbaya TA, Tsoumpou I, Nardo LG (May 2009). "The likelihood of live birth and multiple birth after single versus double embryo transfer at the cleavage stage: a systematic review and meta-analysis". Fertil. Steril. 94 (3): 936–45. doi:10.1016/j.fertnstert.2009.04.003. PMID 19446809.
- ^ a b Maheshwari, A.; Griffiths, S.; Bhattacharya, S. (2010). "Global variations in the uptake of single embryo transfer". Human Reproduction Update 17 (1): 107. doi:10.1093/humupd/dmq028. PMID 20634207.
- ^ Zarutskiea PW, Phillips JA (2007). "Re-analysis of vaginal progesterone as luteal phase support (LPS) in assisted reproduction (ART) cycles". Fertility and Sterility 88 (supplement 1): S113. doi:10.1016/j.fertnstert.2007.07.365.
- ^ Khan N, Richter KS, Blake EJ, et al. Case-matched comparison of intramuscular versus vaginal progesterone for luteal phase support after in vitro fertilization and embryo transfer. Presented at: 55th Annual Meeting of the Pacific Coast Reproductive Society; April 18–22, 2007; Rancho Mirage, CA.
- ^ Goudge CS, Nagel TC, Damario MA (June 2009). "Duration of progesterone-in-oil support after in vitro fertilization and embryo transfer: a randomized, controlled trial". Fertil. Steril. 94 (3): 946–51. doi:10.1016/j.fertnstert.2009.05.003. PMID 19523613.
- ^ a b Teman, Elly. 2010. Birthing a Mother: the Surrogate Body and the Pregnant Self. Berkeley: University of California Press.
- ^ Blakeslee, Sandra (1984-02-04). "Infertile Woman Has Baby Through Embryo Transfer". The New York Times. http://query.nytimes.com/gst/fullpage.html?sec=health&res=9404EEDC143BF937A35751C0A962948260. Retrieved 2010-05-01.
- ^ http://www.humc.edu/calendar/careacc.html
- ^ a b Friedrich, Otto; Constable, Anne; Samghabadi, Raji (1984-09-10). "Medicine: A Legal, Moral, Social Nightmare". Time. http://www.time.com/time/magazine/article/0,9171,952517,00.html. Retrieved 2010-05-01.
- ^ a b "The New Origins of Life". Time. 1984-09-10. http://www.time.com/time/magazine/article/0,9171,952514,00.html. Retrieved 2010-05-01.
- ^ http://www.cdc.gov/ART/
- ^ http://www.obgmanagement.com/srm/pdf/first_live_birth_donation.pdf
- ^ http://www.obgmanagement.com/srm.asp?id=5030
- ^ Embryo Transfer in Cattle Retrieved on 21 October 2008
- ^ Embryo Sexing Technology Retrieved on 21 October 2008
External links
- How embryo transfer works as part of fertility treatment
- The blastocyst transfer process - a form of embryo transfer
- One at a time website - benefits of Single Embryo Transfer
Categories:- Fertility medicine
- In vitro fertilisation
- Cryobiology
- Fertility
Wikimedia Foundation. 2010.

