- Miglustat
-
Miglustat 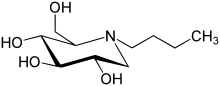
Systematic (IUPAC) name (2R,3R,4R,5S)-1-butyl-2-(hydroxymethyl)piperidine-3,4,5-triol Clinical data AHFS/Drugs.com monograph MedlinePlus a604015 Licence data US FDA:link Pregnancy cat. X(US) Legal status ℞-only (US) Routes Oral Pharmacokinetic data Bioavailability 97% Protein binding Nil Metabolism Nil Half-life 6–7 hours Excretion Renal, unchanged Identifiers CAS number 72599-27-0 
ATC code A16AX06 PubChem CID 51634 DrugBank APRD01118 ChemSpider 46764 
UNII ADN3S497AZ 
ChEBI CHEBI:50381 
ChEMBL CHEMBL1029 
Synonyms 1,5-(butylimino)-1,5-dideoxy-D-glucitol Chemical data Formula C10H21NO4 Mol. mass 219.28 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Miglustat is a drug developed by Actelion and is used primarily to treat Type 1 Gaucher disease (GD1). It is marketed under the trade name Zavesca. Miglustat (OGT 918, N-butyl-deoxynojirimycin) is an imino sugar (molecular weight: 219 daltons), a synthetic analogue of D-glucose and a white to off-white crystalline solid that has a bitter taste. The primary pharmacological activity of miglustat is inhibition of the enzyme glucosylceramide synthase, catalyzing the first step in the biosynthesis of glycosphingolipids (GSL), i.e., the formation of glucosylceramide (GlcCer). Reduced formation of GlcCer will lead to decreased biosynthesis of more complex GSL. This therapeutic principle, called substrate reduction therapy (SRT), may be useful in disorders of intracellular (predominantly lysosomal) accumulation of GSL either due to their deficient breakdown or intracellular transport/trafficking.[1] Miglustat exhibits a large volume of distribution and has the capacity to access deep organs such as the brain, bone and lung.
Miglustat inhibits glucosylceramide synthase,[2] an essential enzyme for the synthesis of most glycosphingolipids. Miglustat is a glucosylceramide synthase inhibitor. It works by inhibitting glucosylceramide synthase (the enzyme that forms glucosylceramide, which accumulates within the macrophages). Miglustat is used to treat adults with mild to moderate type 1 Gaucher disease and it is the first treatment to be approved for patients with Niemann-Pick type C disease. Miglustat may only be used in the treatment of type 1 Gaucher patients for whom enzyme replacement therapy is unsuitable[3] and it's been approved in the European Union for the treatment of progressive neurological manifestations in adult or pediatric patients with Niemann-Pick type C disease (NPC). It has also been approved for NPC treatment in Canada, Switzerland, Brazil, Australia, Turkey and Israel but not in the United States.
Type 1 Gaucher disease is an autosomal recessive disorder one gets from both parents. People with type 1 Gaucher have a defect in the enzyme called glucocerebrosidase (also known as acid β-glucosidase) that acts on a fatty substance glucosylceramide (also known as glucocerebroside). Accumulation of glucosylceramide causes liver and spleen enlargement, changes in the bone marrow and blood, and bone disease. Treatment with miglustat is known as substrate reduction therapy (SRT). Unlike enzyme replacement therapy (ERT), which has a direct effect on the breakdown of glycosphingolipids, the concept of SRT in Gaucher disease involves reduction of the delivery of potential storage material to the macrophage system. Patients treated with miglustat for 3 years show signicant improvements in organ volumes and haematological parameters. Miglustat was effective over time and showed acceptable tolerability in patients who continued with treatment for 3 years.[4] It is also being investigated to treat Tay-sachs disease [5]
In October 2007, Actelion initiated a Phase IIa proof-of-concept clinical trial with miglustat in cystic fibrosis (CF). It is the first time that miglustat is being tested in a clinical setting involving 25 CF patients affected by the specific delF508 mutation.
See also
- Isofagomine tartrate, another orphan drug for the treatment of Gaucher's disease with a similar chemical structure, but a different mechanism of action
References
- ^ http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM196758.pdf
- ^ van Giersbergen PL, Dingemanse J (2007). "Influence of Food Intake on the Pharmacokinetics of Miglustat, an Inhibitor of Glucosylceramide Synthase". The Journal of Clinical Pharmacology 47 (10): 1277–82. doi:10.1177/0091270007305298. PMID 17720777.
- ^ Journal of Inherit Metabol. Diseases, 2003;26:513-526. Cox T et cols. THE ROLE OF THE IMINOSUGAR N-BUTYLDEOXINOJIRIMYCIN (MIGLUSTAT) IN THE MANAGEMENT OF TYPE I (NONNEURONOPATHIC) GAUCHER DISEASE: A POSITION STATEMENT.
- ^ Journal of Inherit Metabol. Diseases, 2004;27:757-766. Elstein et cols. SUSTAINED THERAPEUTIC EFFECTS OF ORAL MIGLUSTAT IN TYPE I GAUCHER DISEASE.
- ^ http://www.clinicaltrials.gov/ct2/show/NCT00672022?term=tay-sachs&rank=3
Other alimentary tract and metabolism products (A16) Amino acids and derivatives Enzymes Carbohydrate metabolism: sucrase (Sacrosidase) • alpha-glucosidase (Alglucosidase alfa)
Glycolipid/sphingolipid: glucocerebrosidase (Alglucerase, Imiglucerase, Velaglucerase alfa) • alpha-galactosidase (Agalsidase alfa, Agalsidase beta)
Glycosaminoglycan: iduronidase (Laronidase) • arylsulfatase B (Galsulfase) • iduronate-2-sulfatase (Idursulfase)Various alimentary tract
and metabolism productsTioctic acid • Anethole trithione • Sodium phenylbutyrate • Nitisinone • Zinc acetate • Miglustat • Sapropterin
This drug article relating to the gastrointestinal system is a stub. You can help Wikipedia by expanding it.
