- Fatty acid
-
Not to be confused with fat.
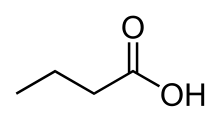 Butyric acid, a short-chain fatty acid
Butyric acid, a short-chain fatty acid
Types of fats in food - Unsaturated fat
- Monounsaturated fat
- Polyunsaturated fat
- Trans fat
- Cis fat
- Omega fatty acids:
- Saturated fat
See also - Fatty acid
- Essential fatty acid
In chemistry, especially biochemistry, a fatty acid is a carboxylic acid with a long unbranched aliphatic tail (chain), which is either saturated or unsaturated. Most naturally occurring fatty acids have a chain of an even number of carbon atoms, from 4 to 28.[1] Fatty acids are usually derived from triglycerides or phospholipids. When they are not attached to other molecules, they are known as "free" fatty acids. Fatty acids are important sources of fuel because, metabolized, they yield large quantities of ATP. Many cell types can use either glucose or fatty acids for this purpose. In particular, heart and skeletal muscle prefer fatty acids. The brain cannot use fatty acids as a source of fuel; it relies on glucose or ketone bodies.[2]
Contents
Types of fatty acids
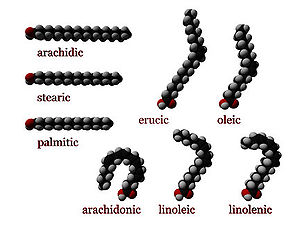
Fatty acids that have double bonds are known as unsaturated. Fatty acids without double bonds are known as saturated. They differ in length as well.
Length of free fatty acid chains
Fatty acid chains differ by length, often categorized as short, medium, or long.
- Short-chain fatty acids (SCFA) are fatty acids with aliphatic tails of fewer than six carbons (i.e. butyric acid.
- Medium-chain fatty acid (MCFA) are fatty acids with aliphatic tails of 6–12[3] carbons, which can form medium-chain triglycerides.
- Long-chain fatty acid (LCFA) are fatty acids with aliphatic tails longer than 12 carbons.[4]
- Very long chain fatty acid (VLCFA) are fatty acids with aliphatic tails longer than 22 carbons
Unsaturated fatty acids
Unsaturated fatty acids have one or more double bonds between carbon atoms. (Pairs of carbon atoms connected by double bonds can be saturated by adding hydrogen atoms to them, converting the double bonds to single bonds. Therefore, the double bonds are called unsaturated.)
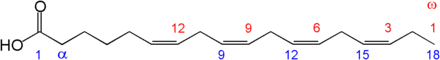
The two carbon atoms in the chain that are bound next to either side of the double bond can occur in a cis or trans configuration.
- cis
- A cis configuration means that adjacent hydrogen atoms are on the same side of the double bond. The rigidity of the double bond freezes its conformation and, in the case of the cis isomer, causes the chain to bend and restricts the conformational freedom of the fatty acid. The more double bonds the chain has in the cis configuration, the less flexibility it has. When a chain has many cis bonds, it becomes quite curved in its most accessible conformations. For example, oleic acid, with one double bond, has a "kink" in it, whereas linoleic acid, with two double bonds, has a more pronounced bend. Alpha-linolenic acid, with three double bonds, favors a hooked shape. The effect of this is that, in restricted environments, such as when fatty acids are part of a phospholipid in a lipid bilayer, or triglycerides in lipid droplets, cis bonds limit the ability of fatty acids to be closely packed, and therefore could affect the melting temperature of the membrane or of the fat.
- trans
- A trans configuration, by contrast, means that the next two hydrogen atoms are bound to opposite sides of the double bond. As a result, they do not cause the chain to bend much, and their shape is similar to straight saturated fatty acids.
In most naturally occurring unsaturated fatty acids, each double bond has three n carbon atoms after it, for some n, and all are cis bonds. Most fatty acids in the trans configuration (trans fats) are not found in nature and are the result of human processing (e.g., hydrogenation).
The differences in geometry between the various types of unsaturated fatty acids, as well as between saturated and unsaturated fatty acids, play an important role in biological processes, and in the construction of biological structures (such as cell membranes).
Examples of Unsaturated Fatty Acids Common name Chemical structure Δx C:D n−x Myristoleic acid CH3(CH2)3CH=CH(CH2)7COOH cis-Δ9 14:1 n−5 Palmitoleic acid CH3(CH2)5CH=CH(CH2)7COOH cis-Δ9 16:1 n−7 Sapienic acid CH3(CH2)8CH=CH(CH2)4COOH cis-Δ6 16:1 n−10 Oleic acid CH3(CH2)7CH=CH(CH2)7COOH cis-Δ9 18:1 n−9 Elaidic acid CH3(CH2)7CH=CH(CH2)7COOH trans-Δ9 18:1 n−9 Vaccenic acid CH3(CH2)5CH=CH(CH2)9COOH trans-Δ11 18:1 n−7 Linoleic acid CH3(CH2)4CH=CHCH2CH=CH(CH2)7COOH cis,cis-Δ9,Δ12 18:2 n−6 Linoelaidic acid CH3(CH2)4CH=CHCH2CH=CH(CH2)7COOH trans,trans-Δ9,Δ12 18:2 n−6 α-Linolenic acid CH3CH2CH=CHCH2CH=CHCH2CH=CH(CH2)7COOH cis,cis,cis-Δ9,Δ12,Δ15 18:3 n−3 Arachidonic acid CH3(CH2)4CH=CHCH2CH=CHCH2CH=CHCH2CH=CH(CH2)3COOHNIST cis,cis,cis,cis-Δ5Δ8,Δ11,Δ14 20:4 n−6 Eicosapentaenoic acid CH3CH2CH=CHCH2CH=CHCH2CH=CHCH2CH=CHCH2CH=CH(CH2)3COOH cis,cis,cis,cis,cis-Δ5,Δ8,Δ11,Δ14,Δ17 20:5 n−3 Erucic acid CH3(CH2)7CH=CH(CH2)11COOH cis-Δ13 22:1 n−9 Docosahexaenoic acid CH3CH2CH=CHCH2CH=CHCH2CH=CHCH2CH=CHCH2CH=CHCH2CH=CH(CH2)2COOH cis,cis,cis,cis,cis,cis-Δ4,Δ7,Δ10,Δ13,Δ16,Δ19 22:6 n−3 Essential fatty acids
Main article: Essential fatty acidFatty acids that are required by the human body but cannot be made in sufficient quantity from other substrates, and therefore must be obtained from food, are called essential fatty acids. There are two series of essential fatty acids: one has a double bond three carbon atoms removed from the methyl end; the other has a double bond six carbon atoms removed from the methyl end. Humans lack the ability to introduce double bonds in fatty acids beyond carbons 9 and 10, as counted from the carboxylic acid side.[5] Two essential fatty acids are linoleic acid (LA) and alpha-linolenic acid (ALA). They are widely distributed in plant oils. The human body has a limited ability to convert ALA into the longer-chain n-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which can also be obtained from fish.
Saturated fatty acids
Saturated fatty acids are long-chain carboxylic acids that usually have between 12 and 24 carbon atoms and have no double bonds. Thus, saturated fatty acids are saturated with hydrogen (since double bonds reduce the number of hydrogens on each carbon). Because saturated fatty acids have only single bonds, each carbon atom within the chain has 2 hydrogen atoms (except for the omega carbon at the end that has 3 hydrogens).
Examples of Saturated Fatty Acids Common name Chemical structure C:D Caprylic acid CH3(CH2)6COOH 8:0 Capric acid CH3(CH2)8COOH 10:0 Lauric acid CH3(CH2)10COOH 12:0 Myristic acid CH3(CH2)12COOH 14:0 Palmitic acid CH3(CH2)14COOH 16:0 Stearic acid CH3(CH2)16COOH 18:0 Arachidic acid CH3(CH2)18COOH 20:0 Behenic acid CH3(CH2)20COOH 22:0 Lignoceric acid CH3(CH2)22COOH 24:0 Cerotic acid CH3(CH2)24COOH 26:0 Nomenclature
Several different systems of nomenclature are used for fatty acids. The following table describes the most common systems.
System Example Explanation Trivial nomenclature Palmitoleic acid Trivial names (or common names) are non-systematic historical names, which are the most frequent naming system used in literature. Most common fatty acids have trivial names in addition to their systematic names (see below). These names frequently do not follow any pattern, but they are concise and often unambiguous. Systematic nomenclature (9Z)-octadecenoic acid Systematic names (or IUPAC names) derive from the standard IUPAC Rules for the Nomenclature of Organic Chemistry, published in 1979,[6] along with a recommendation published specifically for lipids in 1977.[7] Counting begins from the carboxylic acid end. Double bonds are labelled with cis-/trans- notation or E-/Z- notation, where appropriate. This notation is generally more verbose than common nomenclature, but has the advantage of being more technically clear and descriptive. Δx nomenclature cis,cis-Δ9,Δ12 octadecadienoic acid In Δx (or delta-x) nomenclature, each double bond is indicated by Δx, where the double bond is located on the xth carbon–carbon bond, counting from the carboxylic acid end. Each double bond is preceded by a cis- or trans- prefix, indicating the conformation of the molecule around the bond. For example, linoleic acid is designated "cis-Δ9, cis-Δ12 octadecadienoic acid". This nomenclature has the advantage of being less verbose than systematic nomenclature, but is no more technically clear or descriptive. n−x nomenclature n−3 n−x (n minus x; also ω−x or omega-x) nomenclature both provides names for individual compounds and classifies them by their likely biosynthetic properties in animals. A double bond is located on the xth carbon–carbon bond, counting from the terminal methyl carbon (designated as n or ω) toward the carbonyl carbon. For example, α-Linolenic acid is classified as a n−3 or omega-3 fatty acid, and so it is likely to share a biosynthetic pathway with other compounds of this type. The ω−x, omega-x, or "omega" notation is common in popular nutritional literature, but IUPAC has deprecated it in favor of n−x notation in technical documents.[6] The most commonly researched fatty acid biosynthetic pathways are n−3 and n−6, which are hypothesized[by whom?] to decrease or increase, respectively,[citation needed] inflammation. Lipid numbers 18:3
18:3, n−6
18:3, cis,cis,cis-Δ9,Δ12,Δ15Lipid numbers take the form C:D, where C is the number of carbon atoms in the fatty acid and D is the number of double bonds in the fatty acid. This notation can be ambiguous, as some different fatty acids can have the same numbers. Consequently, when ambiguity exists this notation is usually paired with either a Δx or n−x term.[6] Production
Fatty acids are usually produced industrially by the hydrolysis of triglycerides, with the removal of glycerol (see oleochemicals). Phospholipids represent another source. Some fatty acids are produced synthetically by hydrocarboxylation of alkenes.
Free fatty acids
Main article: fatty acid synthesisThe biosynthesis of fatty acids involves the condensation of acetyl-CoA. Since this coenzyme carries a two-carbon-atom group, almost all natural fatty acids have even numbers of carbon atoms.
The "uncombined fatty acids" or "free fatty acids" found in organism[which?] come from the breakdown of a triglyceride[citation needed]. Because they are insoluble in water, these fatty acids are transported (solubilized, circulated) while bound to plasma protein albumin. The levels of "free fatty acid" in the blood are limited by the availability of albumin binding sites.
Fatty acids in dietary fats
The following table gives the fatty acid, vitamin E and cholesterol composition of some common dietary fats.[8] [9]
Saturated Monounsaturated Polyunsaturated Cholesterol Vitamin E g/100g g/100g g/100g mg/100g mg/100g Animal fats Lard 40.8 43.8 9.6 93 0.00 Duck fat[10] 33.2 49.3 12.9 100 2.70 Butter 54.0 19.8 2.6 230 2.00 Vegetable fats Coconut oil 85.2 6.6 1.7 0 .66 Palm oil 45.3 41.6 8.3 0 33.12 Cottonseed oil 25.5 21.3 48.1 0 42.77 Wheat germ oil 18.8 15.9 60.7 0 136.65 Soya oil 14.5 23.2 56.5 0 16.29 Olive oil 14.0 69.7 11.2 0 5.10 Corn oil 12.7 24.7 57.8 0 17.24 Sunflower oil 11.9 20.2 63.0 0 49.0 Safflower oil 10.2 12.6 72.1 0 40.68 Hemp oil 10 15 75 0 Canola/Rapeseed oil 5.3 64.3 24.8 0 22.21 Reactions of fatty acids
Fatty acids exhibit reactions like other carboxylic acid, i.e. they undergo esterification and acid-base reactions.
Acidity
Fatty acids do not show a great variation in their acidities, as indicated by their pKas. Nonanoic acid, for example, has a pKa of 4.96, being only slightly weaker than acetic acid (4.76). As the chain length increases the solubility of the fatty acids in water decreases very rapidly, so that the longer-chain fatty acids have minimal effect on the pH of an aqueous solution. Even those fatty acids that are insoluble in water will dissolve in warm ethanol, and can be titrated with sodium hydroxide solution using phenolphthalein as an indicator to a pale-pink endpoint. This analysis is used to determine the free fatty acid content of fats; i.e., the proportion of the triglycerides that have been hydrolyzed.
Hydrogenation and hardening
Hydrogenation of unsaturated fatty acids is widely practiced to give saturated fatty acids, which are less prone toward rancidification. Since the saturated fatty acids are higher melting that the unsaturated relatives, the process is called hardening. This technology is used to convert vegetable oils into margarine. During partial hydrogenation, unsaturated fatty acids can be isomerized from cis to trans configuration.[11]
More forcing hydrogenation, i.e. using higher pressures of H2 and higher temperatures, converts fatty acids fatty alcohols. Fatty alcohols are, however, more easily produced from fatty acid esters.
In the Varrentrapp reaction certain unsaturated fatty acids are cleaved in molten alkali, a reaction at one time of relevance to structure elucidation.
Auto-oxidation and rancidity
Main article: RancidificationUnsaturated fatty acids undergo a chemical change known as auto-oxidation. The process requires oxygen (air) and is accelerated by the presence of trace metals. Vegetable oils resists this process because they contain antioxidants, such as tocopherol. Fats and oils often are treated with chelating agents such as citric acid to remove the metal catalysts.
Ozonolysis
Unsaturated fatty acids are susceptible to degradation by ozone. This reaction is practiced in the production azelaic acid ((CH2)7(CO2H)2) from oleic acid.[11]
Circulation
Digestion and intake
Main article: Digestion#Fat digestionShort- and medium-chain fatty acids are absorbed directly into the blood via intestine capillaries and travel through the portal vein just as other absorbed nutrients do. However, long-chain fatty acids are not directly released into the intestinal capillaries. Instead they are absorbed into the fatty walls of the intestine villi and reassembled again into triglycerides. The triglycerides are coated with cholesterol and protein (protein coat) into a compound called a chylomicron.
Within the villi, the chylomicron enters a lymphatic capillary called a lacteal, which merges into larger lymphatic vessels. It is transported via the lymphatic system and the thoracic duct up to a location near the heart (where the arteries and veins are larger). The thoracic duct empties the chylomicrons into the bloodstream via the left subclavian vein. At this point the chylomicrons can transport the triglycerides to tissues where they are stored or metabolized for energy.
Metabolism
Fatty acids (provided either by ingestion or by drawing on triglycerides stored in fatty tissues) are distributed to cells to serve as a fuel for muscular contraction and general metabolism. They are consumed by mitochondria to produce ATP through beta oxidation.
Distribution
Main article: Blood fatty acidsBlood fatty acids are in different forms in different stages in the blood circulation. They are taken in through the intestine in chylomicrons, but also exist in very low density lipoproteins (VLDL) and low density lipoproteins (LDL) after processing in the liver. In addition, when released from adipocytes, fatty acids exist in the blood as free fatty acids.
It is proposed that the blend of fatty acids exuded by mammalian skin, together with lactic acid and pyruvic acid, is distinctive and enables animals with a keen sense of smell to differentiate individuals.[12]
See also
- Essential fatty acid
- Fatty acid metabolism
- Fatty acid synthase
- Fatty acid synthesis
- List of saturated fatty acids
- Saturated fat
- Unsaturated fat
- Vegetable oils
References
- ^ IUPAC Compendium of Chemical Terminology (2nd ed.). International Union of Pure and Applied Chemistry. 1997. ISBN 052151150X. http://goldbook.iupac.org/F02330.html. Retrieved 2007-10-31.
- ^ Mary K. Campbell, Shawn O. Farrell (2006). Biochemistry (5th ed.). Cengage Learning. p. 579. ISBN 0534405215.
- ^ Medscape: Free CME, Medical News, Full-text Journal Articles & More
- ^ Christopher Beermann1, J Jelinek1, T Reinecker2, A Hauenschild2, G Boehm1, and H-U Klör2, "Short term effects of dietary medium-chain fatty acids and n-3 long-chain polyunsaturated fatty acids on the fat metabolism of healthy volunteers"
- ^ Cell Biology: A Short Course
- ^ a b c Rigaudy, J.; Klesney, S.P. (1979). Nomenclature of Organic Chemistry. Pergamon. ISBN 0080223699. OCLC 5008199.
- ^ "The Nomenclature of Lipids. Recommendations, 1976". European Journal of Biochemistry 79 (1): 11–21. 1977. doi:10.1111/j.1432-1033.1977.tb11778.x. http://www.blackwell-synergy.com/doi/pdf/10.1111/j.1432-1033.1977.tb11778.x.
- ^ Food Standards Agency (1991). "Fats and Oils". McCance & Widdowson's the Composition of Foods. Royal Society of Chemistry.
- ^ Ted Altar. "More Than You Wanted To Know About Fats/Oils". Sundance Natural Foods Online. http://www.efn.org/~sundance/fats_and_oils.html. Retrieved 2006-08-31.
- ^ U. S. Department of Agriculture.. "USDA National Nutrient Database for Standard Reference". U. S. Department of Agriculture.. http://www.nal.usda.gov/fnic/foodcomp/search/. Retrieved 2010-02-17.
- ^ a b David J. Anneken, Sabine Both, Ralf Christoph, Georg Fieg, Udo Steinberner, Alfred Westfechtel "Fatty Acids" in Ullmann's Encyclopedia of Industrial Chemistry 2006, Wiley-VCH, Weinheim. doi:10.1002/14356007.a10_245.pub2
- ^ "Electronic Nose Created To Detect Skin Vapors". Science Daily. July 21, 2009. http://www.sciencedaily.com/releases/2009/07/090721091839.htm. Retrieved 2010-05-18.
External links
Lipids: fatty acids Saturated VFA: Acetic (C2) · Propionic (C3) · Butyric (C4) · Valeric (C5) · Caproic (C6) · Enanthic (C7) · Caprylic (C8) · Pelargonic (C9) · Capric (C10) · Undecylic (C11) · Lauric (C12) · Tridecylic (C13) · Myristic (C14) · Pentadecanoic (C15) · Palmitic (C16) · Margaric (C17) · Stearic (C18) · Nonadecylic (C19) · Arachidic (C20) · Heneicosylic (C21) · Behenic (C22) · Tricosylic (C23) · Lignoceric (C24) · Pentacosylic (C25) · Cerotic (C26) · Heptacosylic (C27) · Montanic (C28) · Nonacosylic (C29) · Melissic (C30) · Hentriacontylic (C31) · Lacceroic (C32) · Psyllic (C33) · Geddic (C34) · Ceroplastic (C35) · Hexatriacontylic (C36)n−3 Unsaturated n−6 Unsaturated n−9 Unsaturated biochemical families: prot · nucl · carb (glpr, alco, glys) · lipd (fata/i, phld, strd, gllp, eico) · amac/i · ncbs/i · ttpy/i Categories:- Fatty acids
- Nutrition
- Unsaturated fat
Wikimedia Foundation. 2010.