- Adverse drug reaction
-
Adverse drug reaction Classification and external resources 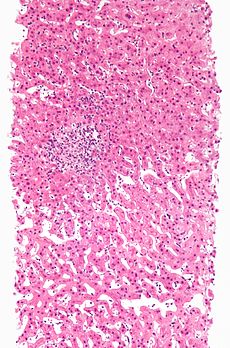
Adverse drug reaction leading to hepatitis (drug-induced hepatitis) with granulomata. Other causes were excluded with extensive investigations. Liver biopsy. H&E stain.ICD-10 T88.7, Y40-Y59 ICD-9 995.2, E850-E858 DiseasesDB 295 MeSH D004362 An adverse drug reaction (abbreviated ADR) is an expression that describes harm associated with the use of given medications at a normal dosage. ADRs may occur following a single dose or prolonged administration of a drug or result from the combination of two or more drugs. The meaning of this expression differs from the meaning of "side effect", as this last expression might also imply that the effects can be beneficial.[1] The study of ADRs is the concern of the field known as pharmacovigilance.
Contents
Classification
ADRs may be classified by e.g. cause and severity.
Cause
- Type A: Augmented pharmacologic effects - dose dependent and predictable
- Intolerance
- Side Effects
- Type B: Bizarre effects (or idiosyncratic) - dose independent and unpredictable
- Type C: Chronic effects
- Type D: Delayed effects
- Type E: End-of-treatment effects
- Type F: Failure of therapy
- typeG: Genetic reactions
Types A and B were proposed in the 1970s,[2] and the other types were proposed subsequently when the first two proved insufficient to classify ADRs.[3]
Seriousness and Severity
The American Food and Drug Administration defines a serious adverse event as one when the patient outcome is one of the following:[4]
- Death
- Life-threatening
- Hospitalization (initial or prolonged)
- Disability - significant, persistent, or permanent change, impairment, damage or disruption in the patient's body function/structure, physical activities or quality of life.
- Congenital anomaly
- Requires intervention to prevent permanent impairment or damage
Severity is a point on an arbitrary scale of intensity of the adverse event in question. The terms "severe" and "serious" when applied to adverse events are technically very different. They are easily confused but can not be used interchangeably, require care in usage.
A headache is severe, if it causes intense pain. There are scales like "visual analog scale" that help us assess the severity. On the other hand, a headache is not usually serious (but may be in case of subarachnoid haemorrhage, subdural bleed, even a migrane may temporally fit criteria), unless it also satisfies the criteria for seriousness listed above.
Overall Drug Risk
While no official scale exists yet to communicate overall drug risk, the iGuard Drug Risk Rating System is a five color rating scale similar to the Homeland Security Advisory System:[5]
- Red (high risk)
- Orange (elevated risk)
- Yellow (guarded risk)
- Blue (general risk)
- Green (low risk)
Location
Adverse effects may be local, i.e. limited to a certain location, or systemic, where a medication has caused adverse effects throughout the systemic circulation.
For instance, some ocular antihypertensives cause systemic effects,[6] although they are administered locally as eye drops, since a fraction escapes to the systemic circulation.
Mechanisms
As research better explains the biochemistry of drug use, fewer ADRs are Type B and more are Type A. Common mechanisms are:
- Abnormal pharmacokinetics due to
- Synergistic effects between either
- a drug and a disease
- two drugs
Abnormal pharmacokinetics
Comorbid disease states
Various diseases, especially those that cause renal or hepatic insufficiency, may alter drug metabolism. Resources are available that report changes in a drug's metabolism due to disease states.[7]
Genetic factors
Abnormal drug metabolism may be due to inherited factors of either Phase I oxidation or Phase II conjugation.[8][9] Pharmacogenomics is the study of the inherited basis for abnormal drug reactions.
Phase I reactions
Inheriting abnormal alleles of cytochrome P450 can alter drug metabolism. Tables are available to check for drug interactions due to P450 interactions.[10][11]
Inheriting abnormal butyrylcholinesterase (pseudocholinesterase) may affect metabolism of drugs such as succinylcholine[12]
Phase II reactions
Inheriting abnormal N-acetyltransferase which conjugated some drugs to facilitate excretion may affect the metabolism of drugs such as isoniazid, hydralazine, and procainamide.[11][12]
Inheriting abnormal thiopurine S-methyltransferase may affect the metabolism of the thiopurine drugs mercaptopurine and azathioprine.[11]
Interactions with other drugs
The risk of drug interactions is increased with polypharmacy.
Protein binding
These interactions are usually transient and mild until a new steady state is achieved.[13][14] These are mainly for drugs without much first-pass liver metabolism. The principal plasma proteins for drug binding are:[15]
- albumin
- α1-acid glycoprotein
- lipoproteins
Some drug interactions with warfarin are due to changes in protein binding.[15]
Cytochrome P450
Patients have abnormal metabolism by cytochrome P450 due to either inheriting abnormal alleles or due to drug interactions. Tables are available to check for drug interactions due to P450 interactions.[10]
Synergistic effects
An example of synergism is two drugs that both prolong the QT interval.
Assessing causality
Causality assessment is used to determine the likelihood that a drug caused a suspected ADR. There are a number of different methods used to judge causation, including the Naranjo algorithm, the Venulet algorithm and the WHO causality term assessment criteria. Each have pros and cons associated with their use and most require some level of expert judgement to apply.[16] An ADR should not be labeled as 'certain' unless the ADR abates with a challenge-dechallenge-rechallenge protocol (stopping and starting the agent in question). The chronology of the onset of the suspected ADR is important, as another substance or factor may be implicated as a cause; co-prescribed medications and underlying psychiatric conditions may be factors in the ADR. A simple scale is available at http://annals.org/cgi/content/full/140/10/795.[1]
Assigning causality to a specific agent often proves difficult, unless the event is found during a clinical study or large databases are used. Both methods have difficulties and can be fraught with error. Even in clinical studies some ADRs may be missed. Psychiatric ADRs are often missed as they are grouped together in the questionnaires used to assess the population [1].
Monitoring bodies
Many countries have official bodies that monitor drug safety and reactions. On an international level, the WHO runs the Uppsala Monitoring Centre, and the European Union runs the European Medicines Agency (EMEA). In the United States, the Food and Drug Administration (FDA) is responsible for monitoring post-marketing studies.
Examples of adverse effects associated with specific medications
Condition Substance Abortion, miscarriage or uterine hemorrhage misoprostol (Cytotec), a labor-inducing drug (this is a case where the adverse effect has been used legally and illegally for performing abortions) Addiction many sedatives and analgesics such as diazepam, morphine, etc. Birth defects Thalidomide and Accutane Bleeding of the intestine aspirin therapy Cardiovascular disease COX-2 inhibitors (i.e. Vioxx) Deafness and kidney failure gentamicin (an antibiotic) Death, following sedation propofol (Diprivan) Dementia heart bypass surgery Depression or hepatic injury interferon Diabetes atypical antipsychotic medications (neuroleptic psychiatric drugs) Diarrhea orlistat (Xenical) Erectile dysfunction many drugs, such as antidepressants Fever vaccination (in the past, imperfectly manufactured vaccines, such as BCG and poliomyelitis, have caused the very disease they intended to fight). Glaucoma corticosteroid-based eye drops Hair loss and anemia chemotherapy against cancer, leukemia, etc. Headache spinal anesthesia Hypertension ephedrine users, which prompted FDA to remove the status of dietary supplement of ephedra extracts Insomnia stimulants, Ritalin, Adderall, etc. Lactic acidosis stavudine (Zerit, for anti-HIV therapy) or metformin (for diabetes) Liver failure paracetamol Melasma and thrombosis estrogen-containing hormonal contraception such as the combined oral contraceptive pill Irreversible Peripheral neuropathy fluoroquinolone medications [17][18][19] Rhabdomyolysis statins (anti-cholesterol drugs) Seizures withdrawal from benzodiazepine Drowsiness or increase in appetite antihistamine use. Some antihistamines are used in sleep aids explicitly because they cause drowsiness. Stroke or heart attack sildenafil (Viagra) when used with nitroglycerine Suicide, increased tendency fluoxetine and other SSRI antidepressants Parkinsonism MPTP a meperidine related drug considered highly neurotoxic Tardive dyskinesia long-term use of metoclopramide and many antipsychotic medications Spontaneous Tendon rupture fluoroquinolone drugs [20] even occurring as late as 6 months after treatment had been terminated.[21] See also
- Classification of Pharmaco-Therapeutic Referrals
- Drug therapy problems
- EudraVigilance (European Union)
- History of pharmacy
- Iatrogenesis
- Paradoxical reaction
- Polypharmacy
- Toxicology
- The Medical Letter on Drugs and Therapeutics
- Yellow Card Scheme (UK)
References
- ^ a b Nebeker JR, Barach P, Samore MH (2004). "Clarifying adverse drug events: a clinician's guide to terminology, documentation, and reporting". Ann. Intern. Med. 140 (10): 795–801. PMID 15148066.
- ^ Rawlins MD, Thompson JW. Pathogenesis of adverse drug reactions. In: Davies DM, ed. Textbook of adverse drug reactions. Oxford: Oxford University Press, 1977:10.
- ^ Aronson JK. Drug therapy. In: Haslett C, Chilvers ER, Boon NA, Colledge NR, Hunter JAA, eds. Davidson's principles and practice of medicine 19th ed. Edinburgh: Elsevier Science, 2002:147-
- ^ "MedWatch - What Is A Serious Adverse Event?". http://www.fda.gov/medwatch/report/DESK/advevnt.htm. Retrieved 2007-09-18.
- ^ Barriaux, Marianne (2007-10-02). "'Traffic-light' medicine risk website to launch". London: The Guardian. http://www.guardian.co.uk/business/2007/oct/02/7. Retrieved 2010-04-23.
- ^ Rang, H. P. (2003). Pharmacology. Edinburgh: Churchill Livingstone. ISBN 0-443-07145-4. Page 146
- ^ "Clinical Drug Use". http://www.clinicaldruguse.com/. Retrieved 2007-09-18.
- ^ Phillips KA, Veenstra DL, Oren E, Lee JK, Sadee W (2001). "Potential role of pharmacogenomics in reducing adverse drug reactions: a systematic review". JAMA 286 (18): 2270–9. doi:10.1001/jama.286.18.2270. PMID 11710893.
- ^ Goldstein DB (2003). "Pharmacogenetics in the laboratory and the clinic". N. Engl. J. Med. 348 (6): 553–6. doi:10.1056/NEJMe020173. PMID 12571264.
- ^ a b "Drug-Interactions.com". http://medicine.iupui.edu/flockhart/. Retrieved 2007-09-18.
- ^ a b c Weinshilboum R; Collins, Francis S.; Weinshilboum, Richard (2003). "Inheritance and drug response". N. Engl. J. Med. 348 (6): 529–37. doi:10.1056/NEJMra020021. PMID 12571261.
- ^ a b Evans WE, McLeod HL (2003). "Pharmacogenomics--drug disposition, drug targets, and side effects". N. Engl. J. Med. 348 (6): 538–49. doi:10.1056/NEJMra020526. PMID 12571262.
- ^ DeVane CL (2002). "Clinical significance of drug binding, protein binding, and binding displacement drug interactions". Psychopharmacology bulletin. 36 (3): 5–21. PMID 12473961.
- ^ Benet LZ, Hoener BA (2002). "Changes in plasma protein binding have little clinical relevance". Clin. Pharmacol. Ther. 71 (3): 115–21. doi:10.1067/mcp.2002.121829. PMID 11907485.OVID full text summary table at OVID
- ^ a b Sands CD, Chan ES, Welty TE (2002). "Revisiting the significance of warfarin protein-binding displacement interactions". The Annals of pharmacotherapy 36 (10): 1642–4. doi:10.1345/aph.1A208. PMID 12369572. http://www.theannals.com/cgi/reprint/36/10/1642.
- ^ Davies EC, Rowe PH, James S et al. (2011). "An Investigation of Disagreement in Causality Assessment of Adverse Drug Reactions". Pharm Med 25 (1): 17–24. doi:10.2165/11539800-000000000-00000. http://adisonline.com/pharmaceuticalmedicine/Fulltext/2011/25010/An_Investigation_of_Disagreement_in_Causality.3.aspx.
- ^ Aoun M, Jacquy C, Debusscher L et al. (July 1992). "Peripheral neuropathy associated with fluoroquinolones". Lancet 340 (8811): 127. doi:10.1016/0140-6736(92)90460-K. PMID 1352007. http://linkinghub.elsevier.com/retrieve/pii/0140-6736(92)90460-K.
- ^ Cohen JS (December 2001). "Peripheral neuropathy associated with fluoroquinolones". Ann Pharmacother 35 (12): 1540–7. doi:10.1345/aph.1Z429. PMID 11793615. http://www.theannals.com/cgi/pmidlookup?view=long&pmid=11793615.
- ^ Hedenmalm K, Spigset O (April 1996). "Peripheral sensory disturbances related to treatment with fluoroquinolones". J. Antimicrob. Chemother. 37 (4): 831–7. doi:10.1093/jac/37.4.831. PMID 8722551. http://jac.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=8722551.
- ^ Jagose JT, McGregor DR, Nind GR, Bailey RR (December 1996). "Achilles tendon rupture due to ciprofloxacin". N. Z. Med. J. 109 (1035): 471–2. PMID 9006634.
- ^ Casparian JM, Luchi M, Moffat RE, Hinthorn D (May 2000). "Quinolones and tendon ruptures". South. Med. J. 93 (5): 488–91. PMID 10832946.
- The Adverse Drug Reaction Electronic System
 United States
United States - Latest Updates on The Adverse Drug Reaction
Consequences of external causes (T66–T78, 990–995) Temperature/radiation reduced temperature: Hypothermia · Immersion foot syndromes (Trench foot • Tropical immersion foot • Warm water immersion foot) · Chilblains · Frostbite · Cold intolerance • Acrocyanosis • Erythrocyanosis crurumradiation: Radiation poisoning · Radiation burn · Chronic radiation keratosis • Eosinophilic, polymorphic, and pruritic eruption associated with radiotherapy • Radiation acne • Radiation cancer • Radiation recall reaction • Radiation-induced erythema multiforme • Radiation-induced hypertrophic scar • Radiation-induced keloid • Radiation-induced morpheaAir Food Maltreatment Emesis Adverse effect Adverse drug reactionOther Ungrouped
skin conditions
resulting from
physical factorsDermatosis neglecta • Pinch mark • Pseudoverrucous papules and nodules • Sclerosing lymphangiitis • Tropical anhidrotic asthenia • UV-sensitive syndromeenvironmental skin conditions: Electrical burn • frictional/traumatic/sports (Black heel and palm • Equestrian perniosis • Jogger's nipple • Pulling boat hands • Runner's rump • Surfer's knots • Tennis toe • Vibration white finger • Weathering nodule of ear • Wrestler's ear • Coral cut • Painful fat herniation ) • Uranium dermatosis
iv use (Skin pop scar • Skin track • Slap mark • Pseudoacanthosis nigricans • Narcotic dermopathy)Drug reactions (Y40-Y59, E930-E949) Adverse drug reaction/
drug eruptionantibiotics: Penicillin drug reaction · Sulfonamide hypersensitivity syndrome · Urticarial erythema multiforme · Adverse effects of fluoroquinolones
hormones: Steroid acne · Steroid folliculitis
chemotherapy: Chemotherapy-induced acral erythema · Chemotherapy-induced hyperpigmentation · Scleroderma-like reaction to taxanes · Hydroxyurea dermopathy · Exudative hyponychial dermatitis
blood: Anticoagulant-induced skin necrosis · Warfarin necrosis · Vitamin K reaction · Texier's disease
anticonvulsant: Anticonvulsant hypersensitivity syndrome
water-balance/acid-base: Allopurinol hypersensitivity syndrome
pulmonary: Leukotriene receptor antagonist-associated Churg–Strauss syndrome
other specified agents: Adverse reaction to biologic agents · Adverse reaction to cytokines · Bromoderma · Halogenoderma · Iododerma · Red man syndrome · Methotrexate-induced papular eruption
unspecified agent: Acute generalized exanthematous pustulosis · Bullous drug reaction · Drug-induced acne · Drug-induced angioedema · Drug-related gingival hyperplasia · Drug-induced lichenoid reaction · Drug-induced lupus erythematosus · Drug-induced nail changes · Drug-induced pigmentation · Drug-induced pseudolymphoma · Drug-induced urticaria · Fixed drug reaction · Stevens–Johnson syndrome · Injection site reaction · Linear IgA bullous dermatosis · Toxic epidermal necrolysis · HIV disease-related drug reaction · Photosensitive drug reaction · Serum sickness-like reactionCategories:- Pharmacy
- Pharmacology
- Drug safety
- Type A: Augmented pharmacologic effects - dose dependent and predictable
Wikimedia Foundation. 2010.
