- Felodipine
-
Felodipine 

Systematic (IUPAC) name 3-ethyl 5-methyl 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate Clinical data Trade names Plendil AHFS/Drugs.com monograph MedlinePlus a692016 Pregnancy cat. C(US) Legal status ℞ Prescription only Routes Oral Pharmacokinetic data Bioavailability 15% [1] Metabolism Hepatic Half-life ?? Excretion Renal Identifiers CAS number 72509-76-3 ATC code C08CA02 PubChem CID 3333 DrugBank APRD00374 ChemSpider 3216 
UNII OL961R6O2C 
KEGG D00319 
ChEBI CHEBI:585948 ChEMBL CHEMBL1480 
Chemical data Formula C18H19Cl2NO4 Mol. mass 384.259 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Felodipine is a calcium channel blocker (calcium antagonist), a drug used to control hypertension (high blood pressure). It is marketed under the brand name Plendil by AstraZeneca and Renedil by Sanofi-Aventis. The formulation patent for the substance expired in 2007.
AstraZeneca dropped Plendil from its support and AZ&Me free Rx access program in October 2008.
Contents
Interactions
Studies dating back to 1989 have suggested that felodipine in combination with grapefruit juice can cause toxic effects. Oral administration of felodipine is first metabolized in the gastrointestinal tract and liver by the enzyme CYP3A4. Grapefruit juice contains bergamottin which is found to have an inhibiting effect over this enzyme and as a result the bioavailability of the drug increases, raising the risk for abnormal side effects.[2]
Contraindications and cautions
contraindicated with allergy to felodipine or other calcium channel blockers, sick sinus syndrome, heart block(second and third degree, lactation. use cautiously with pregnancy, impaired hepatic function.
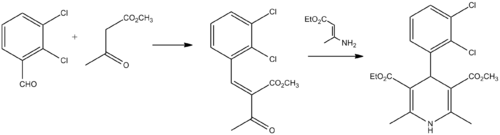
Synthesis
Berntsson, P. B.; Carlsson, A. I.; Gaarder, J. O.; Ljung, B. R.; 1981, U.S. Patent 4,264,611.
References
- ^ AstraZeneca MI Department, 16th April 2010
- ^ Jawad Kiani, Sardar Z Imam (October 30 2007). "Medicinal importance of grapefruit juice and its interaction with various drugs". Nutr J. 6 (33): 33. doi:10.1186/1475-2891-6-33. PMC 2147024. PMID 17971226. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2147024. Retrieved 2008-04-09.
This drug article relating to the cardiovascular system is a stub. You can help Wikipedia by expanding it.