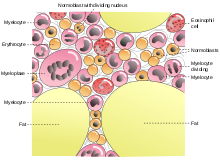
- Hematopoietic stem cell
-
Hematopoietic stem cell 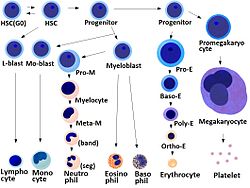
HSC=Hematopoietic stem cell, Progenitor=Progenitor cell, L-blast=Lymphoblast, Lymphocyte, Mo-blast=Monoblast, Monocyte, Myeloblast, Pro-M=Promyelocyte, Myelocyte, Meta-M=Metamyelocyte, Neutrophil, Eosinophil, Basophil, Pro-E=Proerythroblast, Baso-E=Basophilic erythroblast, poly-E=Polychromatic erythroblast, Ortho-E=Orthochromatic erythroblast, Erythrocyte, Promegakaryocyte, Megakaryocyte, Platelet Latin cellula haematopoietica precursoria Code TH H2.00.01.0.00006 Hematopoietic stem cells (HSCs), also spelled Hæmatopoietic stem cells, are multipotent stem cells that give rise to all the blood cell types from the myeloid (monocytes and macrophages, neutrophils, basophils, eosinophils, erythrocytes, megakaryocytes/platelets, dendritic cells), and lymphoid lineages (T-cells, B-cells, NK-cells). The definition of haematopoietic stem cells has undergone considerable revision in the last two decades. The hematopoietic tissue contains cells with long-term and short-term regeneration capacities and committed multipotent, oligopotent, and unipotent progenitors. HSCs constitute 1:10.000 of cells in myeloid tissue.
HSCs are a heterogeneous population. Three classes of stem cells exist, distinguished by their ratio of lymphoid to myeloid progeny (L/M) in blood. Myeloid-biased (My-bi) HSC have low L/M ratio (>0, <3), whereas lymphoid-biased (Ly-bi) HSC show a large ratio (>10). The third category consists of the balanced (Bala) HSC for which 3 ≤ L/M ≤ 10. Much work is currently being undertaken to investigate the properties of these different classes of HSCs, but it appears that only the myeloid-biased and -balanced HSCs have durable self-renewal properties. In addition, serial transplantation experiments have shown that each subtype preferentially re-creates its blood cell type distribution, suggesting an inherited epigenetic program for each subtype.
Contents
Source
HSCs are found in the bone marrow of adults, which includes femurs, hip, ribs, sternum, and other bones. Cells can be obtained directly by removal from the hip using a needle and syringe, or from the blood following pre-treatment with cytokines, such as G-CSF (granulocyte colony-stimulating factors), that induce cells to be released from the bone marrow compartment. Other sources for clinical and scientific use include umbilical cord blood, peripheral blood a small number of stem and progenitor cells circulate in the bloodstream, in the past 10 years, researchers have found that they can coax the cells to migrate from marrow to blood in greater numbers by injecting the donor with a cytokine, such as granulocyte-colony stimulating factor (GCSF)and recent study shown that ex-vivo expansion of HSCs is possible in 3D bioreactor. Because HSCs are not generated in the adult but during the embryogenesis, many scientific groups are studying HSCs during the embryonic development. It is now well described in mammalians that the first definitive HSCs are detected in the AGM (Aorta-gonad-mesonephros), and then massively expanded in the Fetal Liver prior to colonize before birth the bone marrow. Such fundamental research could help to understand the mechanisms that are responsible of HSCs generation and/or amplification, and to the discovery of new molecules that could eventually be used to maintain or expand HSCs in vitro.
Functional characteristics
Multipotency and self-renewal
As stem cells, HSC are defined by their ability to replenish all blood cell types (Multipotency) and their ability to self-renew.
It is known that a small number of HSCs can expand to generate a very large number of daughter HSCs. This phenomenon is used in bone marrow transplantation, when a small number of HSCs reconstitute the hematopoietic system. This indicates that, subsequent to bone marrow transplantation, symmetrical cell divisions into two daughter HSCs must occur.
Stem cell self-renewal is thought to occur in the stem cell niche in the bone marrow, and it is reasonable to assume that key signals present in this niche will be important in self-renewal. There is much interest in the environmental and molecular requirements for HSC self-renewal, as understanding the ability of HSC to replenish themselves will eventually allow the generation of expanded populations of HSC in vitro that can be used therapeutically.
Stem cell heterogeneity
It was originally believed that all HSC were alike in their self-renewal and differentiation abilities. This view was first challenged by the 2002 discovery by the Muller-Sieburg group in San Diego, who illustrated that different stem cells can show distinct repopulation patterns that are epigenetically predetermined intrinsic properties of clonal Thy-1lo SCA-1+ lin- c-kit+ HSC.[1][2][3] The results of these clonal studies led to the notion of lineage bias. Using the ratio ρ = L / M of lymphoid (L) to myeloid (M) cells in blood as a quantitative marker, the stem cell compartment can be split into three categories of HSC. Balanced (Bala) HSC repopulate peripheral white blood cells in the same ratio of myeloid to lymphoid cells as seen in unmanipulated mice (on average about 15% myeloid and 85% lymphoid cells, or 3≤ρ≤10). Myeloid-biased (My-bi) HSC give rise to too few lymphocytes resulting in ratios 0<ρ<3, while lymphoid-biased (Ly-bi) HSC generate too few myeloid cells, which results in lymphoid-to-myeloid ratios of 10<ρ<oo. All three types are norm three types of HSC, and they do not represent stages of differentiation. Rather, these are three classes of HSC, each with an epigenetically fixed differentiation program. These studies also showed that lineage bias is not stochastically regulated or dependent on differences in environmental influence. My-bi HSC self-renew longer than balanced or Ly-bi HSC. The myeloid bias results from reduced responsiveness to the lymphopoetin Interleukin 7 (IL-7).[2]
Subsequent to this, other groups confirmed and highlighted the original findings (refer to the excellent mini-review by Timm Schroeder[4]). For example, the Eaves group confirmed in 2007 that repopulation kinetics, long-term self-renewal capacity, and My-bi and Ly-bi are stably inherited intrinsic HSC properties.[5] In 2010, the Goodell group provided additional insights about the molecular basis of lineage bias in side population Side population (SP) SCA-1+ lin- c-kit+ HSC.[6] As previously shown for IL-7 signaling, it was found that a member of the transforming growth factor family (TGF-beta) induces and inhibits the proliferation of My-bi and Ly-bi HSC, respectively.
Functional assays
A cobblestone area-forming cell (CAFC) assay is a cell culture-based empirical assay. When plated onto a confluent culture of stromal feeder layer, a fraction of HSCs creep between the gaps (even though the stromal cells are touching each other) and eventually settle between the stromal cells and the substratum (here the dish surface) or trapped in the cellular processes between the stromal cells. Emperipolesis is the in vivo phenomenon in which one cell is completely engulfed into another (e.g., thymocytes into thymic nurse cells); on the other hand, when in vitro, lymphoid lineage cells creep beneath nurse-like cells, the process is called pseudoemperipolesis. This similar phenomenon is more commonly known in HSC field by the cell culture terminology cobble stone area-forming cells (CAFC), which means areas of cluster of cells that look dull cobblestone-like under phase contrast microscopy, compared to the other HSCs, which are refractile. This happens because the cells that are floating loosely on top of the stromal cells are spherical and thus refractile. However, the cells that creep beneath the stromal cells are flattened and, thus, not refractile. The mechanism of pseudoemperipolesis is only recently coming to light. It may be mediated by interaction through CXCR4 (CD184) the receptor for CXC Chemokines (e.g., SDF1) and α4β1 integrins.[7]
Mobility
HSCs have a higher potential than other immature blood cells to pass the bone marrow barrier, and, thus, may travel in the blood from the bone marrow in one bone to another bone. If they settle in the thymus, they will develop into T cells. In the case of fetuses and other extramedullary hematopoiesis, HSCs may also settle in the liver or spleen and develop.
This ability is the reason why HSCs may be harvested directly from the blood.
Physical characteristics
With regard to morphology, hematopoietic stem cells resemble lymphocytes. They are non-adherent, and rounded, with a rounded nucleus and low cytoplasm-to-nucleus ratio. Since PHSC cannot be isolated as a pure population, it is not possible to identify them in a microscope. The above description is based on the morphological characteristics of a heterogeneous population, of which PHSC are a component.
Markers
In reference to phenotype, hematopoeitic stem cells are identified by their small size, lack of lineage (lin) markers, low staining (side population) with vital dyes such as rhodamine 123 (rhodamineDULL, also called rholo) or Hoechst 33342, and presence of various antigenic markers on their surface.
Cluster of differentiation and other markers
Many of these markers belong to the cluster of differentiation series, like: CD34, CD38, CD90, CD133, CD105, CD45, and also c-kit, - the receptor for stem cell factor. The haematopoietic stem cells are negative for the markers that are used for detection of lineage commitment, and are, thus, called Lin-; and, during their purification by FACS, a bunch of up to 14 different mature blood-lineage marker, e.g., CD13 & CD33 for myeloid, CD71 for erythroid, CD19 for B cells, CD61 for megakaryocytic, etc. for humans; and, B220 (murine CD45) for B cells, Mac-1 (CD11b/CD18) for monocytes, Gr-1 for Granulocytes, Ter119 for erythroid cells, Il7Ra, CD3, CD4, CD5, CD8 for T cells, etc. (for mice) antibodies are used as a mixture to deplete the lin+ cells or late multipotent progenitors (MPP)s.
There are many differences between the human and mice hematopoietic cell markers for the commonly accepted type of haematopoietic stem cells.[1].
- Mouse HSC : CD34lo/-, SCA-1+, Thy1.1+/lo, CD38+, C-kit+, lin-
- Human HSC : CD34+, CD59+, Thy1/CD90+, CD38lo/-, C-kit/CD117+, lin-
However, not all stem cells are covered by these combinations that, nonetheless, have become popular. In fact, even in humans, there are hematopoietic stem cells that are CD34-/CD38-.[8][9] Also some later studies suggested that earliest stem cells may lack c-kit on the cell surface.[10] For human HSCs use of CD133 was one step ahead as both CD34+ and CD34- HSCs were CD133+.
Traditional purification method used to yield a reasonable purity level of mouse haematopoietic stem cells, in general, requires a large(~10-12) battery of markers, most of which were surrogate markers with little functional significance, and thus partial overlap with the stem cell populations and sometimes other closely related cells that are not stem cells. Also, some of these markers (e.g., Thy1) are not conserved across mouse species, and use of markers like CD34- for HSC purification requires mice to be at least 8 weeks old.
SLAM code
Alternative methods that could give rise to similar or better harvest of stem cells is a hot area of research and are presently emerging. One such method uses a signature of SLAM family of cell surface molecules. SLAM (Signaling lymphocyte activation molecule) family is a group of >10 molecules whose genes are located mostly tandemly in a single locus on chromosome 1 (mouse), all belonging to a subset of immunoglobulin gene superfamily, and originally thought to be involved in T-cell stimulation. This family includes CD48, CD150, CD244, etc., CD150 being the founding member, and, thus, also called slamF1, i.e., SLAM family member 1.
The signature SLAM code for the hemopoietic hierarchy are:
- Hematopoietic stem cells (HSC) : CD150+CD48-CD244-
- Multipotent progenitor cells (MPPs) : CD150-CD48-CD244+
- Lineage-restricted progenitor cells (LRPs) : CD150-CD48+CD244+
- Common myeloid progenitor (CMP) : lin-SCA-1-c-kit+CD34+CD16/32mid
- Granulocyte-macrophage progenitor (GMP) : lin-SCA-1-c-kit+CD34+CD16/32hi
- Megakaryocyte-erythroid progenitor (MEP) : lin-SCA-1-c-kit+CD34-CD16/32low
For HSCs, CD150+CD48- was sufficient instead of CD150+CD48-CD244- because CD48 is a ligand for CD244, and both would be positive only in the activated lineage-restricted progenitors. It seems that this code was more efficient than the more tedious earlier set of the large number of markers, and are also conserved across the mouse strains; however, recent work has shown that this method excludes a large number of HSCs and includes an equally large number of non-stem cells.[11] .[12] CD150+CD48- gave stem cell purity comparable to Thy1loSCA-1+lin-c-kit+ in mice.[13]
LT-HSC/ST-HSC/Early MPP/Late MPP
Irving Weissman's group at Stanford University was the first to isolate mouse hematopoietic stem cells in 1988 and was also the first to work out the markers to distinguish the mouse long-term (LT-HSC) and short-term (ST-HSC) haematopoietic stem cells (self-renew-capable), and the Multipotent progenitors (MPP, low or no self-renew capability — the later the developmental stage of MPP, the lesser the self-renewal ability and the more of some of the markers like CD4 and CD135):
- LT-HSC : CD34-, SCA-1+, Thy1.1+/lo, C-kit+, lin-, CD135-, Slamf1/CD150+
- ST-HSC : CD34+, SCA-1+, Thy1.1+/lo, C-kit+, lin-, CD135-, Slamf1/CD150+, Mac-1 (CD11b)lo
- Early MPP : CD34+, SCA-1+, Thy1.1-, C-kit+, lin-, CD135+, Slamf1/CD150-, Mac-1 (CD11b)lo, CD4lo
- Late MPP : CD34+, SCA-1+, Thy1.1-, C-kit+, lin-, CD135high, Slamf1/CD150-, Mac-1 (CD11b)lo, CD4lo
Nomenclature of hematopoietic colonies and lineages
Between 1948 and 1950, the Committee for Clarification of the Nomenclature of Cells and Diseases of the Blood and Blood-forming Organs issued reports on the nomenclature of blood cells.[14][15] An overview of the terminology is shown below, from earliest to final stage of development:
- [root]blast
- pro[root]cyte
- [root]cyte
- meta[root]cyte
- mature cell name
The root for CFU-E is "rubri", for CFU-GM is "granulo" or "myelo" and "mono", for CFU-L is "lympho" and for CFU-Meg is "megakaryo". According to this terminology, the stages of red blood cell formation would be: rubriblast, prorubricyte, rubricyte, metarubricyte, and erythrocyte. However, the following nomenclature seems to be, at present, the most prevalent:
Committee "lympho" "rubri" "granulo" or "myelo" "mono" "megakaryo" Lineage Lymphoid Myeloid Myeloid Myeloid Myeloid CFU CFU-L CFU-GEMM→CFU-E CFU-GEMM→CFU-GM→CFU-G CFU-GEMM→CFU-GM→CFU-M CFU-GEMM→CFU-Meg Process lymphocytopoiesis erythropoiesis granulocytopoiesis monocytopoiesis thrombocytopoiesis [root]blast Lymphoblast Proerythroblast Myeloblast Monoblast Megakaryoblast pro[root]cyte Prolymphocyte Polychromatophilic erythrocyte Promyelocyte Promonocyte Promegakaryocyte [root]cyte - Normoblast Eosino/neutro/basophilic myelocyte Megakaryocyte meta[root]cyte Large lymphocyte Reticulocyte Eosinophilic/neutrophilic/basophilic metamyelocyte, Eosinophilic/neutrophilic/basophilic band cell Early monocyte - mature cell name Small lymphocyte Erythrocyte granulocytes (Eosino/neutro/basophil) Monocyte thrombocytes (Platelets) Osteoclasts also arise from haemopoietic cells of the monocyte/neutrophil lineage, specifically CFU-GM.
Colony-forming units
There are various kinds of colony-forming units:
- Colony-forming unit lymphocyte (CFU-L)
- Colony-forming unit erythrocyte (CFU-E)
- Colony-forming unit granulo-monocyte (CFU-GM)
- Colony-forming unit megakaryocyte (CFU-Me)
- Colony-forming unit Basophil (CFU-B)
- Colony-forming unit Eosinophil (CFU-Eo)
The above CFUs are based on the lineage. Another CFU, the colony-forming unit–spleen (CFU–S) was the basis of an in vivo clonal colony formation, which depends on the ability of infused bone marrow cells to give rise to clones of maturing haematopoietic cells in the spleens of irradiated mice after 8 to 12 days. It was used extensively in early studies, but is now considered to measure more mature progenitor or Transit Amplifying Cells rather than stem cells.
HSC Repopulation Kinetics
Hematopoietic stem cells (HSC) cannot be easily observed directly, and, therefore, their behaviors need to be inferred indirectly. Clonal studies are likely the closest technique for single cell in vivo studies of HSC. Here, sophisticated experimental and statistical methods are used to ascertain that, with a high probability, a single HSC is contained in a transplant administered to a lethally irradiated host. The clonal expansion of this stem cell can then be observed over time by monitoring the percent donor-type cells in blood as the host is reconstituted. The resulting time series is defined as the repopulation kinetic of the HSC.
The reconstitution kinetics are very heterogeneous. However, using symbolic dynamics, one can show that they fall into a limited number of classes.[16] To prove this, several hundred experimental repopulation kinetics from clonal Thy-1lo SCA-1+ lin- c-kit+ HSC were translated into symbolic sequences by assigning the symbols "+", "-", "~" whenever two successive measurements of the percent donor-type cells have a positive, negative, or unchanged slope, respectively. By using the Hamming distance, the repopulation patterns were subjected to cluster analysis yielding 16 distinct groups of kinetics. To finish the empirical proof, the Laplace add-one approach was used to determine that the probability of finding kinetics not contained in these 16 groups is very small. By corollary, this result shows that the haematopoietic stem cell compartment is also heterogeneous by dynamical criteria.
See also
References
- ^ Muller-Sieburg CE, Cho RH, Thoman M, Adkins B, Sieburg HB, Deterministc regulation of haematopoietic stem cell self-renewal and differentiation. Blood. 2002; 100; 1302-9
- ^ a b Muller-Sieburg CE, Cho RH, Karlson L, Huang JF, Sieburg HB. Myeloid-biased hematopoietic stem cells have extensive self-renewal capacity but generate diminished progeny with impaired IL-7 responsiveness. Blood. 2004; 103:4111-8
- ^ Sieburg HB, Cho RH, Dykstra B, Eaves, CJ, Muller-Sieburg, CE. The haematopoietic stem cell compartment consists of a limited number of discrete stem cell subsets. Blood. 2006; 107:2311-6. Epub 2005 Nov 15
- ^ Schroeder, T. Haematopoietic Stem Cell Heterogeneity: Subtypes, Not Unpredictable Behavior. Cell Stem Cell 2010. DOI 10.1016/j.stem.2010.02.006
- ^ Dykstra, B et al. Long-Term Propagation of Distinct Hematopoietic Differentiation Programs In Vivo. Cell Stem Cell, Volume 1, Issue 2, 218-229, 16 August 2007
- ^ Challen, G., Boles, NC, Chambers, SM, Goodell, MA. Distinct Haematopoietic Stem Cell Subtypes Are Differentially Regulated by TGF-beta1. Cell Stem Cel 2010. DOI 10.1016/j.stem.2010.02.002
- ^ Burger JA, Spoo A, Dwenger A, Burger M, Behringer D. CXCR4 chemokine receptors (CD184) and alpha4beta1 integrins mediate spontaneous migration of human CD34+ progenitors and acute myeloid leukaemia cells beneath marrow stromal cells (pseudoemperipolesis). Br J Haematol. 2003 Aug;122(4):579-89. PMID: 12899713
- ^ Bhatia, M., D. Bonnet, B. Murdoch, O.I. Gan and J.E. Dick, A newly discovered class of human haematopoietic cells with SCID-repopulating activity, 4(9), 1038, 1998.
- ^ Guo, Yalin, Lubbert, Michael, Engelhardt, Monika CD34- Hematopoietic Stem Cells: Current Concepts and Controversies Stem Cells 2003; 21: 15-20; First published online ; doi=10.1634/stemcells.21-1-15
- ^ H. Doi et al. (1997) Proc. Natl. Acad. Sci. USA 94, 2513–2517
- ^ David C Weksberg, Stuart M Chambers, Nathan C Boles, and Margaret A Goodell CD150 negative Side Population cells represent a functionally distinct population of long-term haematopoietic stem cells. Blood 2007 : blood-2007-09-115006v1
- ^ Gary Van Zant Stem cell markers: less is more! Blood 107: 855-856.
- ^ Kiel et al., Cell, Vol. 121, 1109–1121, July 1, 2005, Copyright ©2005 by Elsevier Inc. DOI 10.1016/j.cell.2005.05.026
- ^ "First report of the Committee for Clarification of the Nomenclature of Cells and Diseases of the Blood and Blood-forming Organs". Amer J Clin Pathol 18: 443–450. 1948.
- ^ "Third, fourth and fifth reports of the committee for clarification of the nomenclature of cells and diseases of the blood and blood-forming organs". Am J Clin Pathol 20 (6): 562–79. 1950. PMID 15432355.
- ^ Sieburg, HB, Muller-Sieburg, CE. Classification of short kinetics by shape. In Silico Biol. 2004;4(2):209-17
Additional images
External links
- MeSH Hematopoietic+stem+cells
- hemocytoblast at eMedicine Dictionary
Human cell types / list derived primarily from mesoderm Paraxial muscle: Myoblast → Myocyte · Myosatellite cell · Tendon cell · Cardiac muscle cell
adipose: Lipoblast → AdipocyteDigestive systemIntermediate Urinary system (RSC)Angioblast → Endothelial cell · Mesangial cell (Intraglomerular, Extraglomerular) · Juxtaglomerular cell · Macula densa cell
Stromal cell → Interstitial cell → Telocytes
Simple epithelial cell → Podocyte · Kidney proximal tubule brush border cellLateral plate/
hemangioblastBlood/immune
(HSC)see lymphocytessee myeloid cellsMyeloid lineage - Blood (WBC and RBC) Cellular/
HSCsCFU-GMHistiocytes · Kupffer cells · Alveolar macrophage · Microglia · Osteoclasts · Epithelioid cells · giant cells (Langhans giant cells, Foreign-body giant cell, Touton giant cells)CFU-DLCommonCFU-BasoCFU-EosCFU-MegCFU-ECFU-MastNoncellular Blood: Lymphocytes Lymphoid/
HSC:CFU-LLymphopoiesis Cellular differentiation: Stem cells / progenitor cell Sources/types Cell potency Totipotent (Zygote, Spore, Morula) · Pluripotent (Embryonic stem cell, Callus) · Multipotent (Progenitor cell: Endothelial stem cell, Hematopoietic stem cell, Mesenchymal stem cell, Neural stem cell) · Unipotent (Precursor cell)Related articles Stem cell treatments · Stem cell controversy · Stem cell line · Stem cell laws · Stem cell laws and policy in the United StatesCategories:- Hematopoietic stem cells
- Stem cells
- Blood cells
- Leukocytes
Wikimedia Foundation. 2010.