- Cycloaddition
-
A cycloaddition is a pericyclic chemical reaction, in which "two or more unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity."[1] The resulting reaction is a cyclization reaction.
Cycloadditions are usually described by the backbone size of the participants. This would make the Diels-Alder reaction a [4 + 2]cycloaddition, the 1,3-dipolar cycloaddition a [3 + 2]cycloaddition and cyclopropanation of a carbene with an alkene a [2+1]cycloaddition. This type of reaction is non-polar addition reaction.
Contents
Reaction mechanism
Thermal cycloadditions usually have (4n + 2) π electrons participating in the starting material, for some integer n. These occur, for reasons of orbital symmetry, in a suprafacial-suprafacial or antarafacial-antarafacial manner (rare). There are a few examples of thermal cycloadditions which have 4n π electrons (for example the [2 + 2] cycloaddition); these proceed in a suprafacial-antarafacial sense, such as the dimerisation of ketene, in which the orthogonal set of p orbitals allows the reaction to proceed via a crossed transition state.
Cycloadditions in which 4n π electrons participate can also occur as a result of photochemical activation. Here, one component has an electron promoted from the HOMO (π bonding) to the LUMO (π* antibonding). Orbital symmetry is then such that the reaction can proceed in a suprafacial-suprafacial manner. An example is the DeMayo reaction. Another example is shown below, the photochemical dimerization of cinnamic acid.[2][3]
Note that not all photochemical (2+2) cyclizations are cycloadditions; some are known to operate by radical mechanisms.
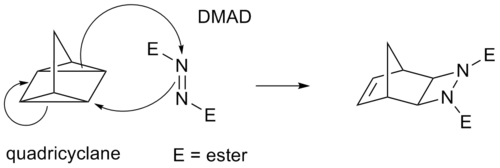
Some cycloadditions instead of π bonds operate through strained cyclopropane rings; as these have significant π character. For example, an analog for the Diels-Alder reaction is the quadricyclane-DMAD reaction:
In the (i+j+...) cycloaddition notation i and j refer to the number of atoms involved in the cycloaddition. In this notation a Diels-Alder reaction is a (4+2)cycloaddition and a 1,3-dipolar addition such as the first step in ozonolysis is a (3+2)cycloaddition. The IUPAC preferred notation however, with [i+j+...] takes electrons into account and not atoms. In this notation the DA reaction and the dipolar reaction both become a [4+2]cycloaddition. The reaction between norbornadiene and an activated alkyne is a [2+2+2]cycloaddition.
Types of cycloaddition
Diels-Alder reactions
The Diels-Alder reaction is a [4+2]cycloaddition reaction.
Huisgen cycloadditions
The Huisgen cycloaddition reaction is a [2+3]cycloaddition.
Nitrone-olefin cycloaddition
The Nitrone-olefin cycloaddition is a [3+2]cycloaddition.
Formal cycloadditions
Cycloadditions often have metal-catalyzed and stepwise radical analogs, however these are not strictly speaking pericyclic reactions. When in a cycloaddition charged or radical intermediates are involved or when the cycloaddition result is obtained in a series of reaction steps they are sometimes called formal cycloadditions to make the distinction with true pericyclic cycloadditions.
One example of a formal [3+3]cycloaddition between a cyclic enone and an enamine catalyzed by n-butyllithium is a Stork enamine / 1,2-addition cascade reaction:[4]
See also
- [4+3] cycloaddition
- [6+4] cycloaddition
References
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Cycloaddition".
- ^ Hein, Sara M. (June 2006). "An Exploration of a Photochemical Pericyclic Reaction Using NMR Data". Journal of Chemical Education 83 (6): 940–942. doi:10.1021/ed083p940.
- ^ The two trans alkenes react head-to-tail, and the isolated isomer is called truxillic acid
- ^ Movassaghi, Mohammad; Bin Chen (2007). "Stereoselective Intermolecular Formal [3+3] Cycloaddition Reaction of Cyclic Enamines and Enones". Angew. Chem. Int. Ed. 46 (4): 565–568. doi:10.1002/anie.200603302. PMID 17146819.
Categories:- Cycloadditions
- Reaction mechanisms
Wikimedia Foundation. 2010.

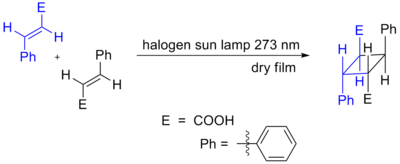


![Intermolecular Formal [3+3] Cycloaddition Reaction](/pictures/enwiki/54/600px-3%2B3-cycloaddition.svg.png)