- Cuprate
-
For cuprate superconductors, see High-temperature_superconductivity#Cuprates.
 The unit cell of high-temperature cuprate superconductor BSCCO-2212
The unit cell of high-temperature cuprate superconductor BSCCO-2212
Cuprates (from Latin cuprum meaning copper) are chemical compounds containing copper anion. Cuprates have been known for centuries and are widely used in inorganic and organic chemistry. However, interest in them has significantly increased since 1986 after the discovery of high-temperature superconductivity in a lanthanum barium copper oxide by Georg Bednorz and Karl Müller.[1] More than 100,000 scientific papers were published on superconductivity in cuprates between 1986 and 2001,[2] and Bednorz and Müller were awarded the Nobel Prize in Physics only a year after their discovery.[3] From 1986 to 2008, almost all known high temperature superconductors were cuprate superconductors and the highest confirmed, ambient-pressure, superconducting transition temperature (Tc) was 135 K achieved in a layered cuprate HgBa2Ca2Cu3Ox in 1993.[4] [5]
Contents
Terminology
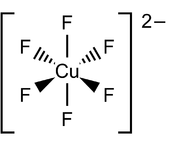
When a copper compound involved in a larger coordination complex has an overall negative charge, then it is referred to as a "cuprate", for example, amminepentachlorocuprate(II) [NH3CuCl5]2–. However, tetraamminedichlorocopper(II) (NH3)4CuCl2 has a neutral charge and therefore is not a cuprate ion.[6]
Inorganic cuprates
Cuprate anions form complexes with negatively charged ligands such as cyanide, hydroxide, or halides. Typical representatives of these complexes are tetracyanocuprate(I), [Cu(CN)4]3–, tetrachlorocuprate(II) [CuCl4]2– and hexahydroxocuprate(II) [Cu(OH)6]4–. There are also rare copper(III) and copper(IV) complexes such as the hexafluorocuprate(III) [CuF6]3– or hexafluorocuprate(IV) [CuF6]2–, which are strong oxidizing agents. Whereas tetracyanocuprate(I) [Cu(CN)4]3- is colorless,[7] most other copper(I) complexes are red-brown; copper(II) complexes have intense turquoise blue color while copper(III) and copper(IV) complexes are orange-red.[8] Dilithium tetrachlorocuprate (Li2CuCl4) is an effective catalyst for the couplings of alkyl halides in the Grignard reaction. It is prepared by mixing lithium chloride (LiCl) and copper(II) chloride (CuCl2) in tetrahydrofuran.[9]
Copper II oxide slightly dissolves in concentrated alkali to form the corresponding cuprate salts (X = alkali metal):
- 3 XOH + CuO + H2O → X3[Cu(OH)6]
Organic cuprates
Main article: Organocopper compoundCuprates play an important role in organic chemistry. The first organocopper compound, the explosive copper(I) acetylide Cu2C2, was synthesized by Rudolf Christian Böttger in 1859.[10]
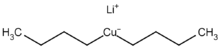
Organic cuprates usually contain an R2Cu moiety, where R is a carbon containing unit. They are rather reactive towards oxygen and water forming copper(I) oxide; they are generally insoluble in inert solvents, tend to be thermally unstable and are difficult to handle. Nevertheless, organocopper reagents are frequently used in organic chemistry as alkylating reagents prepared in situ in an inert environment.[11] The electronegativity of copper is much higher than its neighbor in the group 12 elements, zinc, suggesting less nucleophilicity for carbon.
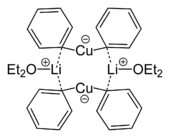 Skeletal formula of a dimer from the crystal structure of lithium diphenylcuprate etherate, Ph2CuLi·2OEt2[12]
Skeletal formula of a dimer from the crystal structure of lithium diphenylcuprate etherate, Ph2CuLi·2OEt2[12]
Ionic cuprates and superconductivity
Main article: High-temperature_superconductivity#CupratesIonic cuprate contain copper in a rare oxidation state 3+, such as CuO–
2. Typical examples are yttrium barium copper oxide (YBCO) and bismuth strontium calcium copper oxide (BSCCO, see top figure for structure), which are the most popular high-temperature superconducting materials. Although such oxides were known for decades, only in 1986, superconductivity was discovered by Georg Bednorz and Karl Müller in a lanthanum barium copper oxide. The transition temperature in their material BaxLa5-xCu5O5(3–y) was "only" Tc = 35 K, this finding stimulated worldwide research on the synthesis and superconductivity in cuprates. Next year, the Tc value was increased to 90 K in the famous YBa2Cu3O7 (YBCO) cuprate.[13] That was a breakthrough achievement because the new superconductors could be cooled by relatively cheap liquid nitrogen rather than expensive liquid helium. Soon after, superconductivity was found in bismuth strontium calcium copper oxide (BSCCO or Bi2Sr2CanCun+1O2n+6-d) with Tc = 95–107 K depending on the n value. Thallium barium calcium copper oxide (TBCCO, TlmBa2Can−1CunO2n+m+2+δ) was the next class of high-Tc cuprate superconductors with Tc = 127 K observed in Tl2Ba2Ca2Cu3O10 (TBCCO-2223) in 1988.[14]In 1993, the transition temperature reached 135 K in a layered cuprate HgBa2Ca2Cu3Ox which remains the highest confirmed ambient-pressure Tc value. Whereas the low-temperature superconductivity in most conventional materials was well explained by the BCS theory (Nobel Prize in Physics in 1972), the mechanism of the high-temperature superconductivity in cuprates remains unsolved.[4][5]
More than 100,000 scientific papers were published on superconductivity in cuprates between 1986 and 2001,[2] and Bednorz and Müller were awarded the Nobel Prize in Physics in 1987.[3] All superconducting cuprates are layered materials having a complex structure described as a superlattice of superconducting CuO2 layers separated by spacer layers where the misfit strain between different layers and dopants in the spacers induce a complex heterogeneity that in the superstripes scenario is intrinsic for high temperature superconductivity. BSCCO superconductors already have large-scale applications. For example, tens of kilometers of BSCCO-2223 electrical cables are being used in the Large Hadron Collider – the world's largest and highest-energy particle accelerator.[15]
-
The Meissner effect demonstrated by levitating a magnet above a cuprate superconductor, which is cooled by liquid nitrogen.
-
For practical applications, BSCCO is compressed with silver metal into a tape via the PIT process
See also
- Benedict's reagent
- Corey-House synthesis
- category:copper compounds
Copper compounds References
- ^ J.G. Bednorz and K.A. Mueller (1986). "Possible high TC superconductivity in the Ba-La-Cu-O system". Z. Phys. B64 (2): 189–193. Bibcode 1986ZPhyB..64..189B. doi:10.1007/BF01303701.
- ^ a b Mark Buchanan (2001). "Mind the pseudogap". Nature 409 (6816): 8–11. doi:10.1038/35051238. PMID 11343081.
- ^ a b Nobel prize autobiography
- ^ a b Lee, Patrick A (2008). "From high temperature superconductivity to quantum spin liquid: progress in strong correlation physics". Reports on Progress in Physics 71: 012501. Bibcode 2008RPPh...71a2501L. doi:10.1088/0034-4885/71/1/012501.
- ^ a b Chu, C. W.; Gao, L.; Chen, F.; Huang, Z. J.; Meng, R. L.; Xue, Y. Y. (1993). "Superconductivity above 150 K in HgBa2Ca2Cu3O8+δ at high pressures". Nature 365 (6444): 323. Bibcode 1993Natur.365..323C. doi:10.1038/365323a0.
- ^ G. Burton (2000). Chemical ideas. Heinemann. p. 268. ISBN 0435631209. http://books.google.com/books?id=ibFdfzSOdHYC&pg=PA268.
- ^ Thomas J. Bruno, Paris D. N. Svoronos (2004). Handbook of basic tables for chemical analysis. CRC Press. p. 543. ISBN 084931573. http://books.google.com/books?id=AFY2gKwh4AsC&pg=PA543.
- ^ Egon Wiberg, Nils Wiberg, Arnold Frederick Holleman (2001). Inorganic chemistry. Academic Press. pp. 1252–1264. ISBN 0123526515. http://books.google.com/books?id=vEwj1WZKThEC&pg=PA1264.
- ^ Atta-ur-Rahman (2002). Bioactive natural products. Elsevier. pp. 73;81;83. ISBN 0444510044. http://books.google.com/books?id=P8B3KEpkqxIC&pg=PA81.
- ^ R. C. Böttger (1859). "Ueber die Einwirkung des Leuchtgases auf verschiedene Salzsolutionen, insbesondere auf eine ammoniakalische Kupferchlorürlösung". Annalen 109 (3): 351. doi:10.1002/jlac.18591090318.
- ^ Louis S. Hegedus (1999). Transition metals in the synthesis of complex organic molecules. University Science Books. pp. 61–65. ISBN 1891389041. http://books.google.com/books?id=101pNv3QpEIC&pg=PA61.
- ^ Lorenzen, Nis Peter (1990). "Synthesis and Structure of a Dimeric Lithium Diphenylcuprate:[{Li(OEt)2}(CuPh2)]2". Angewandte Chemie International Edition in English 29: 300. doi:10.1002/anie.199003001.
- ^ K. M. Wu et al. (1987). "Superconductivity at 93 K in a new mixed-phase Yb-Ba-Cu-O compound system at ambient pressure". Phys. Rev. Lett. 58 (9): 908–910. Bibcode 1987PhRvL..58..908W. doi:10.1103/PhysRevLett.58.908. PMID 10035069.
- ^ Sheng, Z. Z.; Hermann A. M. (1988). "Bulk superconductivity at 120 K in the Tl–Ca/Ba–Cu–O system". Nature 332 (6160): 138–139. Bibcode 1988Natur.332..138S. doi:10.1038/332138a0.
- ^ "HTS materials for LHC current leads". CERN. http://at-mel-cf.web.cern.ch/at-mel-cf/html/HTS_materials.htm.
Categories:- Copper compounds
- Superconductivity
- Copper alloys
- Superconductors
Wikimedia Foundation. 2010.