- Epidural
-
This article is about the anaesthetic technique. For the anatomical site, see Epidural space. For other uses, see Epidural (disambiguation).
- Did you mean Epidural hematoma, a type of traumatic brain injury?
Epidural Intervention
An epidural catheter after insertion. The site has been prepared with tincture of iodine. Depth markings may be seen along the shaft of the catheter.MeSH D000767 The term epidural is often short for epidural analgesia, a form of regional analgesia involving injection of drugs through a catheter placed into the epidural space. The injection can cause both a loss of sensation (anaesthesia) and a loss of pain (analgesia), by blocking the transmission of signals through nerves in or near the spinal cord.
The epidural space is the space inside the bony spinal canal but outside the membrane called the dura mater (sometimes called the "dura"). In contact with the inner surface of the dura is another membrane called the arachnoid mater ("arachnoid"). The arachnoid encompasses the cerebrospinal fluid that surrounds the spinal cord.
Contents
Difference from spinal anaesthesia
Spinal anaesthesia is a technique whereby a local anaesthetic drug is injected into the cerebrospinal fluid. This technique has some similarity to epidural anaesthesia, and the two techniques may be easily confused with each other. Differences include:
- The involved space is larger for an epidural, and subsequently the injected dose is larger, being about 10-20 ml in epidural anesthesia compared to 1.5-3.5 ml in a spinal.
- In an epidural, an indwelling catheter may be placed that avails for additional injections later, while a spinal is usually one-shot only; though a continuous spinal can also be administered, especially in pain management (with morphine pumps).
- The onset of analgesia is approximately 15–30 minutes in an epidural, while it is approximately 5 minutes in a spinal.
- An epidural usually doesn't cause significant neuromuscular block at the lower effective analgesic dosages, while a spinal more often does.
- An epidural may be given at a thoracic or lumbar site, while a spinal must be injected below L2 to avoid piercing and consequently damaging the spinal cord.
- With epidural, it is possible to create segmental blocks as opposed to spinal where the block involves all segments below the highest level of anesthesia.
- The extension of the block with epidural anesthesia is highly dependent on the volume and rate of injection. The position of the patient has little to no impact on the level of the block whereas With spinal anesthesia, the density of the solution combined with the position of the patient influences the level of the block significantly.
Consequently, epidural is safer if a higher level of block is required.
Indications
Injecting medication into the epidural space is primarily performed for analgesia. This may be performed using a number of different techniques and for a variety of reasons. Additionally, some of the side-effects of epidural analgesia may be beneficial in some circumstances (e.g., vasodilation may be beneficial if the patient has peripheral vascular disease). When a catheter is placed into the epidural space (see below) a continuous infusion can be maintained for several days, if needed. Epidural analgesia may be used:
- For analgesia alone, where surgery is not contemplated. An epidural for pain relief (e.g. in childbirth) is unlikely to cause loss of muscle power, but is not usually sufficient for surgery.
- As an adjunct to general anaesthesia. The anaesthetist may use epidural analgesia in addition to general anaesthesia. This may reduce the patient's requirement for opioid analgesics. This is suitable for a wide variety of surgery, for example gynaecological surgery (e.g. hysterectomy), orthopaedic surgery (e.g. hip replacement), general surgery (e.g. laparotomy) and vascular surgery (e.g. open aortic aneurysm repair). See also caudal epidural, below.
- As a sole technique for surgical anaesthesia. Some operations, most frequently Caesarean section, may be performed using an epidural anaesthetic as the sole technique. Typically the patient would remain awake during the operation. The dose required for anaesthesia is much higher than that required for analgesia.
- For post-operative analgesia, after an operation where the epidural was used as either the sole anesthetic, or was used in combination with general anesthesia. Analgesics are given into the epidural space for a few days after surgery, provided a catheter has been inserted. Through the use of a patient-controlled epidural analgesia (PCEA) infusion pump, a patient has the ability to give an occasional extra dose of post-surgical pain medications administered through the epidural.
- For the treatment of back pain. Injection of analgesics and steroids into the epidural space may improve some forms of back pain. See below.
- For the treatment of chronic pain or palliation of symptoms in terminal care, usually in the short- or medium-term.
The epidural space is more difficult and risky to access as one ascends the spine, so epidural techniques are most suitable for analgesia for the chest, abdomen, pelvis or legs. They are (usually) much less suitable for analgesia for the neck, or arms and are not possible for the head (since sensory innervation for the head arises directly from the brain via cranial nerves rather than from the spinal cord via the epidural space.)
Anatomy
Main article: Epidural spaceThe diagram at right depicts the various structures of the spinal column. The spinal cord (yellow core) is in intimate contact with the pia mater (blue). The arachnoid (red) exists superficial to the pia mater, and is attached to it by many trabeculae, giving it a spider-like appearance. This space (light blue) is filled with cerebrospinal fluid (CSF) and is called the subarachnoid space. Superficial to the arachnoid is the dura mater (pink) and although they are unattached, they are kept firmly pressed against one another because of pressure exerted by the CSF. Superficial to the dura mater is a space (pale green), known as the epidural space, that exists between it and the internal surfaces of the vertebral bones and their supporting ligamentous structures. This space is likewise pressed closed by surrounding tissue pressure, so it is called a 'potential' space. The vertebral bones (taupe) are attached to one another by the interspinous ligaments (teal). Insertion of an epidural involves threading a needle between the bones, through the ligaments and into the epidural potential space taking great care to avoid puncturing the layer immediately below containing CSF under pressure.
Technique of insertion
Epidural anaesthesia requires a high level of technical proficiency to avoid serious complications, and should always be performed by a trained anaesthetist or interventional radiologist under image guidance, using a strict aseptic technique to reduce the risk of infection.
Position of the patient
The patient may be in the sitting or lateral position (lying on one side or prone[1]). The sitting patient is asked to slouch and bend forward slightly from the waist to increase the curvature of the spine. The patient lying on the side is asked to draw the knees up to the chin for the same reason. If prone, a pillow is used to cause the back to arch.
Insertion site
The anaesthetist palpates the patient's back and identifies a suitable anatomical gap between the bony spinous processes prior to the procedure. The level of the spine at which the catheter is best placed depends mainly on the site and type of an intended operation or the anatomical origin of pain. The iliac crests are commonly used for reference in order to locate the L4 vertebra, which is well below the termination of the spinal cord. Since innervation of the chest and abdomen travels under the ribs, the anaesthetist can palpate along the corresponding rib to determine placement of the catheter tip.
Most commonly, the anaesthetist conducting an epidural places the catheter in the mid-lumbar, or lower back region of the spine, although occasionally a catheter is placed in the thoracic (chest) or cervical (neck) region. In adults, the spinal cord terminates around the level of the disc between L1 and L2(in neonates it extends to L3 but can reach as low as L4), below which lies a bundle of nerves known as the cauda equina ("horse's tail"). Hence, lumbar epidurals carry a very low risk of injuring the spinal cord.
Locating the epidural space
The skin is infiltrated with local anaesthetic such as lidocaine over the identified space. The insertion point is usually in the midline, although other approaches, such as the paramedian approach, may occasionally be employed. In the paramedian approach, the needle tip passes along a shelf of vertebral bone called the lamina until just before reaching the ligamentun flavum and the epidural space. 'Walking' the needle tip off this lamina allows the clinician to be confident that they are close to the epidural space. This is particularly important in the thoracic spine, where the spinal cord is larger (than in the lumbar spine) and nearly fills the spinal canal increasing the risk of dural puncture and cord damage.
A particular type of needle known as a Tuohy needle is almost invariably used. This needle was specially designed for locating the epidural space safely, and has several specific features for this purpose.
The Tuohy needle is inserted to the ligamentum flavum, is attached to a syringe in the peripheral end, and slowly advanced between two spinous processes. The loss of resistance to injection technique is used to identify the epidural space. This technique is to apply constant pressure on the piston of the syringe towards the barrel as if unfusing, and the loss of resistance is where it is be possible to inject through the syringe, so the piston can easily move into the barrel. This technique works because the ligamentum flavum is extremely dense, and injection into it is almost impossible. In the epidural space, on the other hand, there is negative or neutral pressure.
The syringe may contain air or saline. The principles are the same, but the specifics of the technique are different due to the greater compressibility of air with respect to saline.
Loss of resistance indicates a high likelihood that the tip of the needle has entered the epidural space. A sensation of "pop" or "click" may be felt as the needle breaches the ligamentum flavum just before entering the epidural space. A technique involving constant application of pressure to identify the epidural space whilst advancing the Tuohy needle was described as Dogliotti's principle in 1933. An innovative technique for teaching this sensation of 'loss of resistance' using a banana was described by Leighton in Anesthesiology 70:368-9; 1989 - "A greengrocer's model of the epidural space."[2]
Traditionally anesthesiologist have used either air or saline for identifying the epidural space, depending on their personal preference. However, evidence is accumulating that saline may result in more rapid and satisfactory quality of analgesia.[3][4] In addition to the loss of resistance technique, realtime observation of the advancing needle is becoming more common. This may be done using a portable ultrasound scanner, or with fluoroscopy (moving X-ray pictures).[5]
Feeding the catheter
After placement of the tip of the Tuohy needle into the epidural space the catheter is threaded through the needle. The needle is then withdrawn over the catheter. Generally the catheter is then withdrawn slightly so that 4–6 cm remains in the epidural space.[6] The catheter has depth markings on it (see photo) so that the length of catheter in the epidural space can be estimated.
The catheter is a fine plastic tube, down which anaesthetics may be given into the epidural space. Early catheters had a hole at the end ("end-hole catheters"), but were prone to blockage. More modern catheters ("side-hole catheters") have a blind end but three or more side-holes along the shaft near the tip. This not only disperses the anaesthetic more widely around the catheter, but lessens the likelihood of blockage.
The catheter is typically secured to the skin with adhesive tape or dressings to prevent it becoming dislodged.
In some unusual instances, it may not be required to insert a catheter into the epidural space, e.g. for steroid injections; see below. The anesthesiologist may inject medication into the epidural space through the needle, then remove the needle.
Anaesthetic drugs
A patient receiving an epidural for pain relief typically receives a combination of local anesthetics and opioids. This combination works better than either type of drug used alone. Common local anesthetics include lidocaine, bupivacaine, ropivacaine, and chloroprocaine. Common opioids include morphine, fentanyl, sufentanil, and pethidine (known as meperidine in the U.S.). These are injected in relatively small doses.
Occasionally other agents may be used, such as clonidine or ketamine.
Bolus or infusion?
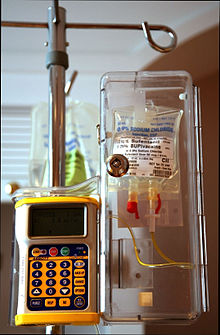 Epidural infusion pump with opioid (sufentanil) in a locked box
Epidural infusion pump with opioid (sufentanil) in a locked box
For a short procedure, the anaesthetist may introduce a single dose of medication (the "bolus" technique). This will eventually wear off. Thereafter, the anaesthetist may repeat the bolus provided the catheter remains undisturbed.
For a prolonged effect, a continuous infusion of drugs may be employed. A common solution for epidural infusion in childbirth or for post-operative analgesia is 0.2% ropivacaine or 0.125% bupivacaine, with 2 μg/mL of fentanyl added. This solution is infused at a rate between 4 and 14 mL/hour, following a loading dose to initiate the nerve block.
There is some evidence that an automated intermittent bolus technique provides better analgesia than a continuous infusion technique, though the total doses are identical.[7][8][9]
Block height and intensity
Typically, the effects of the epidural are noted below a specific level on the body (dermatome). This level (the "block height") is chosen by the anaesthetist. The level is usually 3-4 dermatomes higher than the point of insertion. A very high insertion level may result in sparing of very low dermatomes. For example, a thoracic epidural may be performed for upper abdominal surgery, but may not have any effect on the perineum (area around the genitals) or bladder.[10] Nonetheless, giving very large volumes into the epidural space may spread the block both higher and lower.
The intensity of the block is determined by the concentration of local anaesthetic drugs used. For example, 15 ml 0.1% bupivacaine may provide good analgesia for a woman in labour, but would likely be insufficient for surgery. Conversely, 15 ml of 0.5% bupivacaine would provide a more intense block, likely sufficient for surgery. Since the volume used in each case is the same, the spread of drug, and hence the block height, is likely to be similar.
Removing the catheter
The catheter is usually removed when the patient is able to take oral pain medications. Catheters can safely remain in place for several days with little risk of bacterial infection,[11][12][13] particularly if the skin is prepared with a chlorhexidine solution.[14] Subcutaneously tunneled epidural catheters may be left in place for longer periods, with a low risk of infection or other complications.[15][16][17] Before removing the catheter, the anticoagulation status of the patient must be checked. If the patient is on anticoagulation therapy, it must be stopped for at least 12 hours before the catheter is removed. After removing the catheter, anticoagulation may be resumed after a wait of 2 hours.
Extensions or alternative techniques
Combined spinal-epidurals
Main article: Combined spinal and epidural anaesthesiaFor some procedures, the anaesthetist may choose to combine the rapid onset and reliable, dense block of a spinal anaesthetic with the post-operative analgesic effects of an epidural. This is called combined spinal and epidural anaesthesia (CSE).
The anaesthetist may insert the spinal anaesthetic at one level, and the epidural at an adjacent level. Alternatively, after locating the epidural space with the Tuohy needle, a spinal needle may be inserted through the Tuohy needle into the subarachnoid space. The spinal dose is then given, the spinal needle withdrawn, and the epidural catheter inserted as normal. This method, known as the "needle-through-needle" technique, may be associated with a slightly higher risk of placing the catheter into the subarachnoid space.
Caudal epidurals
The epidural space may be entered through the sacrococcygeal membrane, using a 22g catheter-over-needle or regular 21G needle. Injecting a volume of 1 cc/kg of local anaesthetic here provides good analgesia of the perineum and groin areas. This is typically a single-injection technique and a catheter is not normally placed. This is known as a caudal epidural or "caudal".
The caudal epidural is an effective and safe analgesic technique in children undergoing groin, pelvic or lower extremity surgery. It is usually combined with general anaesthesia since children cannot tolerate the injection awake.
Epidural steroid injections
An epidural steroid injection injection may be used to help reduce the pain and inflammation caused by herniated disc, degenerative disc disease, or spinal stenosis.
Benefits of epidural analgesia after surgery
Epidural analgesia has been demonstrated to have several benefits after surgery. These include:
- Effective analgesia without the need for systemic opioids.[18]
- The incidence of postoperative respiratory problems and chest infections is reduced.[19]
- The incidence of postoperative myocardial infarction ("heart attack") is reduced.[20][21]
- The stress response to surgery is reduced.[20][22]
- Motility of the intestines is improved by blockade of the sympathetic nervous system.[20][23]
- Use of epidural analgesia during surgery reduces blood transfusion requirements.[20]
Despite these benefits, no survival benefit has been proven for high-risk patients.[24]
Potential problems
Side effects
In addition to blocking the nerves which carry pain, local anaesthetic drugs in the epidural space will block other types of nerves as well, in a dose-dependent manner. Depending on the drug and dose used, the effects may last only a few minutes or up to several hours. Epidural typically involves using the opiates fentanyl or sufentanil, with bupivacaine, Fentanyl is a powerful opioid with a potency 80 times that of morphine and side effects common to the opiate class. Sufentanil is another opiate, 5 to 10Xs more potent than Fentanyl. Bupivacaine is markedly toxic, causing excitation: nervousness, tingling around the mouth, tinnitus, tremor, dizziness, blurred vision, or seizures, followed by depression: drowsiness, loss of consciousness, respiratory depression and apnea. Bupivacaine has caused several deaths by cardiac arrest when epidural anesthetic has been accidentally inserted into vein instead of epidural space in the spine. Epidural correctly administered results in three main effects:
- Loss of other modalities of sensation (including touch, and proprioception)
- Loss of muscle power (hence, a risk of falling)
- Loss of function of the sympathetic nervous system, which controls blood pressure
Pain nerves are most sensitive to the effects of the epidural. This means that a good epidural can provide analgesia without affecting muscle power or other types of sensation. The larger the dose used, the more likely it is that the side-effects will be problematic.
For example, a laboring woman may have a continuous epidural during labor that in 85% of cases provides good analgesia without impairing her ability to move around in bed. If she requires a Caesarean section, she is given a larger dose of epidural bupivacaine. After a few minutes, she can no longer move her legs, or feel her abdomen. If her blood pressure drops below 80/50 she is given an intravenous bolus of ephedrine or phenylephrine infusion to compensate. During the operation, she feels no pain.
Very large doses of epidural anaesthetic can cause paralysis of the intercostal muscles and diaphragm (which are responsible for breathing), and loss of sympathetic function to the heart itself, causing a profound drop in heart rate and blood pressure. This requires emergency treatment, and in severe cases may require airway support. This happens because the epidural is blocking the heart's sympathetic nerves, as well as the phrenic nerves, which supply the diaphragm.
It is considered safe practice for all patients with epidurals to be confined to bed to prevent the risk of falls.
The sensation of needing to urinate is diminished, which often requires the placement of a urinary catheter for the duration of the epidural
Opioid drugs in the epidural space are relatively safe (as well as effective). However, very large doses may cause troublesome itch, and rarely, delayed respiratory depression.[25][26][27][28]
Relative contraindications
There are circumstances where the risks of an epidural are higher than normal. These circumstances include:
- Anatomical abnormalities, such as spina bifida or scoliosis
- Previous spinal surgery (where scar tissue may hamper the spread of medication, or may cause an acquired tethered spinal cord)
- Certain problems of the central nervous system, including multiple sclerosis or syringomyelia
- Certain heart-valve problems (such as aortic stenosis, where the vasodilation induced by the anesthetic may impair blood supply to the thickened heart muscle.)
Absolute contraindications
Circumstances in which epidurals should not be used:
- Lack of consent
- Bleeding disorder (coagulopathy) or anticoagulant medication (e.g. warfarin) - risk of spinal cord-compressing hematoma
- Infection near the point of intended insertion
- Infection in the bloodstream which may "seed" via the catheter into the (otherwise relatively impervious) central nervous system
- Uncorrected hypovolemia (low circulating blood volume)
- Allergy to the anaesthetic
Complications and questions about epidural use
These include:
- No pain relief, also called "block failure," occurs in about 5% of patients, while another 15% experience partial relief, sometimes colloquially called a "patchy blockade." If pain relief is inadequate, another epidural may be attempted. Note that regardless of the effect of the epidural on pain relief, once an epidural is administered, many hospitals do not allow patients to get out of bed. A fully effective epidural would prevent a patient from walking at all, however, so this is only a matter of interest in failed attempts. Modern epidural procedures can be done in some hospitals without compromising the mobility of the patient. This is generally referred to as a "walking epidural" and is actually a combination of a traditional epidural block with a spinal injection.[29] Walking is generally encouraged postoperatively in ERAS protocol, once the effect of the epidural has diminished to the point where the patient has enough muscle strength to remain upright.[30]
- The following factors are associated with no pain relief, or block failure with epidurals:[31]
- Obesity
- Multiparity
- History of a previous failure of epidural anesthesia
- History of regular opiate use
- Cervical dilation of more than 7 cm at insertion
- The use of air to find the epidural space while inserting the epidural instead of alternatives like N2O, saline or lidocaine
- The following factors are associated with no pain relief, or block failure with epidurals:[31]
- Accidental dural puncture with headache (common, about 1 in 100 insertions[32][33][34]). The epidural space in the adult lumbar spine is only 3-5mm deep, which means it is comparatively easy to cross it and accidentally puncture the dura (and arachnoid) with the needle. This may cause cerebrospinal fluid (CSF) to leak out into the epidural space, which may in turn cause a post dural puncture headache (PDPH). This can be severe and last several days, and in some rare cases weeks or months. It is caused by a reduction in CSF pressure and is characterised by postural exacerbation when the patient raises their head above the lying position. If severe it may be successfully treated with an epidural blood patch (a small amount of the patient's own blood given into the epidural space via another epidural needle which clots and seals the leak). Most cases resolve spontaneously with time. A change in headache pattern (e.g., headache worse when you lie down) should alert the physician to the possibility of development of rare but dangerous complications, such as subdural hematoma or cerebral venous thrombosis.[35]
- Delayed onset of breastfeeding and shorter duration of breastfeeding: In a study looking at breastfeeding 2 days after epidural anesthesia, epidural analgesia in combination with oxytocin infusion caused women to have significantly lower oxytocin and prolactin levels in response to the baby breastfeeding on day 2 postpartum, which means less milk is produced. Most women with epidurals end up with pitocin augmentation because the epidural slows down the labor.[36]
- Bloody tap (about 1 in 30-50). It is easy to injure an epidural vein with the needle. In patients who have normal blood clotting, it is extremely rare (e.g. 1 in 100,000) for problems to develop. However, in a patient who has a coagulopathy, the patient may be at risk of epidural hematoma. If blood comes back down the needle, the anesthesiologist will normally place the epidural at another level.
- Catheter misplaced into a vein (uncommon, less than 1 in 300). Occasionally the catheter may be misplaced into an epidural vein, which results in all the anaesthetic being injected intravenously, where it can cause seizures or cardiac arrest[37][38] in large doses (about 1 in 10,000 insertions[34]). This also results in block failure.
- High block, as described above (uncommon, less than 1 in 500).
- Catheter misplaced into the subarachnoid space (rare, less than 1 in 1000). If the catheter is accidentally misplaced into the subarachnoid space (e.g. after an unrecognised accidental dural puncture), normally cerebrospinal fluid can be freely aspirated from the catheter (which would usually prompt the anaesthetist to withdraw the catheter and resite it elsewhere). If, however, this is not recognised, large doses of anaesthetic may be delivered directly into the cerebrospinal fluid. This may result in a high block, or, more rarely, a total spinal, where anaesthetic is delivered directly to the brainstem, causing unconsciousness and sometimes seizures.
- Neurological injury lasting less than 1 year (rare, about 1 in 6,700).[39]
- Epidural abscess formation (very rare, about 1 in 145,000).[39] Infection risk increases with the duration catheters are left in place, although infection was still uncommon after an average of 3 to 5 days' duration.[1][2]
- Epidural haematoma formation (very rare, about 1 in 168,000).[39]
- Neurological injury lasting longer than 1 year (extremely rare, about 1 in 240,000).[39]
- Paraplegia (1 in 250,000).[40]
- Arachnoiditis (extremely rare, fewer than 1000 cases in the past 50 years)[41]
- Death (extremely rare, less than 1 in 100,000).[40]
The figures above relate to epidurals in healthy individuals.
There is no evidence to support the concern that epidural analgesia increases the risk of anastomotic breakdown following bowel surgery.[23][42]
Controversial Claims:- The claim that "epidurals significantly slow labor" is considered controversial. This claim is explored in greater detail in further sections of the topic. The following are a few plausible hypotheses for this phenomenon:[citation needed]
- The release of oxytocin, which stimulates the uterine contractions of labor that are needed to move the child out through the birth canal, may be decreased with epidurals due to factors involving the reduction of stress, such as:
- While an epidural is primarily used to alleviate pain during labor, it may also alleviate some of the stress[citation needed], thereby diminishing the release of epinephrine (a natural product of the adrenal glands located atop the kidneys; generally released in association with high levels of stress).[43]
- Diminished release of epinephrine slows the release of oxytocin, because the adrenal glands are controlled by the hypothalamus rather than by hormones.[44]
- Diminished blood pressure, accommodated by both decreased stress and less adrenal release, may decrease the release of oxytocin as a natural mechanism to avoid hypotension.[45] It may also affect the heart-rate of the fetus.[29]
- While an epidural is primarily used to alleviate pain during labor, it may also alleviate some of the stress[citation needed], thereby diminishing the release of epinephrine (a natural product of the adrenal glands located atop the kidneys; generally released in association with high levels of stress).[43]
- The release of oxytocin, which stimulates the uterine contractions of labor that are needed to move the child out through the birth canal, may be decreased with epidurals due to factors involving the reduction of stress, such as:
- Still plausible (though less studied without a documented reproduction in a laboratory setting) are the effects of the reclined position of the woman on the fetus, both immediately prior to and during delivery.[citation needed]
- These hypotheses generally posit an interaction with the force of gravity on fetal position and movement, as demonstrated by the following examples:[citation needed]
- Transverse or posterior fetal positioning may become more likely as a result of the shift in orientation to gravitational force.[citation needed]
- Diminished gravitational assistance is present in building pressure for commencing delivery and for progressing the fetus along the birth canal.[citation needed]
- It is important to note that the orientation of the fetus can be established by ultrasonic stenography prior to, during, and after the administration of an epidural block. This would seem a fine experiment for testing the first hypothesis. It should also be noted that the majority of fetal movement through the birth canal is accomplished by cervical contractions, and so the role of gravity and its force relative to the position of the woman in labor (on delivery, not development) is difficult to establish.[citation needed]
- These hypotheses generally posit an interaction with the force of gravity on fetal position and movement, as demonstrated by the following examples:[citation needed]
- There has been a good deal of concern, based on older observational studies, that women who have epidural analgesia during labor are more likely to require a cesarean delivery.[46] However, the preponderance of evidence now supports the conclusion that the use of epidural analgesia during labor does not have a significant effect on rates of cesarean delivery. A Cochrane review of 20 trials involving a total of 6534 women estimated that the relative risk of cesarean delivery with epidural analgesia as compared with other methods or with no analgesia was 1.07 (95% confidence interval, 0.93 to 1.23).31 Epidural analgesia does increase the duration of the second stage of labor by 15 to 30 minutes and may increase the rate of instrument-assisted vaginal deliveries as well as that of oxytocin administration.[47][48] Clinicians and patients have also been concerned about whether the use of epidural analgesia in early labor increases the risk of cesarean delivery. Three randomized, controlled trials showed that early initiation of epidural analgesia (cervical dilatation, <4 cm) does not increase the rate of cesarean delivery among women with spontaneous or induced labor, as compared with early initiation of analgesia with parenteral opioids.[49][50][51]
Epidural analgesia in childbirth
Safety and efficacy
Epidural analgesia is a relatively safe method of relieving pain in labor. It provides rapid pain relief in most cases. It is more effective than nitrous oxide, opioids, TENS, and other common modalities of analgesia in childbirth.[52] Epidural clonidine has been studied extensively for management of analgesia during labor.[53]
Prolonged labour and risk of instrumental delivery
Epidural analgesia is associated with longer labor.[52] Some researchers claim that it is correlated with an increased chance of operational intervention. The clinical research data on this topic is conflicting. For example, a study in Australia (Roberts, Tracy, Peat, 2000) concluded that having an epidural reduced the woman's chances of having a vaginal birth, without further interventions (such as episiotomy, forceps, ventouse or caesarean section) from 71.4% to 37.8%. Conversely, a 2001 study by researchers at the National Institute of Child Health and Human Development and a 2002 study by researchers at Cornell University and the University of Ontario demonstrated that epidurals do not increase the likelihood of a caesarean section, however the later one confirmed higher frequency of instrumental delivery and fewer and both of them confirmed prolongation of second stage. In 2005, a meta-analysis of 21 studies also showed that epidurals do not increase the likelihood of caesarean section, but they do increase the chance of a forceps or ventouse delivery by 40% (Anim-Somuah, Cochrane Review, 2005).[54] However, the 2000 and 2005 Cochrane Review Meta-Analyses were both driven by a single study by Sharma and colleagues which was based upon a hospital where the caesarean section rate was roughly 1/3 of the national average. This facility's physicians presumably prefer vaginal deliveries and avoid caesarean sections. Because of how low the caesarean section rates were in this facility, the data could not have shown a difference in caesarean rates between epidural and other methods of anagelsia. In fact, when the study is adjusted for this and other statistical issues, the rate of caesarean section is 259% (95% CI 129% to 523%) with epidural versus other analgesics.[55] The COMET Study, published in The Lancet in 2001 (vol358, No9275 p19-23) showed that a combined spinal epidural in labor may speed up the labor process by a few minutes, although those women receiving an epidural had a caesarean rate of 28% and only 35% had a normal birth without instrument assisted delivery.[56]
These differing outcomes may be explained by data that demonstrates that the likelihood of increased intervention is directly related to the quality of the institution or practitioner providing the care: epidurals administered at top-rated institutions do not generally result in a clinically significant increase in caesarean rates, whereas the risk of caesarean delivery at poorly ranked facilities seems to increase with the use of epidural[57]
Effects on the baby
One study concluded that women whose epidurals contain the drug fentanyl were less likely to fully breastfeed their infant in the few days after birth and more likely to stop breastfeeding in the first 24 weeks.[58] However, this study has been criticised for several reasons, one of which is that the original patient records were not examined in this study, and so many of the epidurals were assumed to contain fentanyl when almost certainly they would not have.[59] In addition, all patients who used epidurals in labor had also used systemic pethidine, which would be much more likely to be the cause of any effect on breastfeeding due to the higher amounts of medication used via that route. If that were the case, then early epidurals which avoided the need for pethidine may actually improve breastfeeding outcomes, not worsen them.
Historical notes
In 1901, the use of anaesthesics via the epidural space was first reported, mostly for the treatment of urological diseases but not for surgical procedures. Several techniques were developed in the following years, but never became popular for surgical purposes: most institutions made the transition from a slight sedation to twilight sleep to heavy sedation to general anaesthesia.[60] Caesarean sections under general anesthesia were used only as an emergency measure. In 1912, German physicians found that the injection of an anesthetic, at the base of the spinal cord, would prevent pain impulses from reaching the brain. For expectant mothers, the injection "only reduced the pangs of childbirth; it did not eliminate them," wrote Dr. Morris Fishbein in the March 1943 issue of Hygeia, and a single nerve blocking injection was used only toward the end of labor.[61]
In the 1921, Spanish military surgeon Fidel Pagés developed the modern technique of lumbar epidural anaesthesia, which was popularised in the 1930s by Italian surgery professor Achile Mario Dogliotti.[60]
Dr. Robert A. Hingson, Dr. Waldo B. Edwards, and Dr. James L. Southworth working at the United States Marine Hospital at Stapleton, on Staten Island, New York, developed the technique of continuous caudal anesthesia.[61] Drs. Hingson and Southworth combined the concepts of caudal analgesia and the spinal injection in an operation to strip the varicose veins of a Scottish merchant seaman. The surgeons experimented with a continuous infusion of the local anesthetic, rather than removing the needle after the injection, to originate "continuous caudal analgesia". Dr. Hingson then collaborated with Dr. Edwards, the chief obstetrician at the Marine Hospital, to study the use of this technique in childbirth. The two studied the caudal region to determine where a needle could be safely placed to deliver anesthesia to the spinal nerves without placing the drugs into the spinal fluid.
Testing on a human being did not occur until January 6, 1942, when the wife of a Coast Guardsman was brought into the Marine Hospital for a delivery. Because the woman suffered from rheumatic heart disease, general anesthesia could not be safely used for an emergency Caesarean section, and it was believed that she would not survive the stress of labor. With the use of continuous local anesthesia, the woman and her baby survived. According to Dr. Fishbein's article in Hygeia, a total of 589 women in more than twenty participating hospitals gave birth relatively painlessly in 1942.[62]
The results were published in the January 23, 1943, issue of the Journal of the American Medical Association.[63]
References
- ^ "Epidural Steroid Injections". Pain Management Specialists. http://www.painmanagementsb.com/services/epidural/.
- ^ Leighton BL (February 1989). "A greengrocer's model of the epidural space". Anesthesiology 70 (2): 368–9. doi:10.1097/00000542-198902000-00038. PMID 2913877.
- ^ Norman D (December 2003). "Epidural analgesia using loss of resistance with air versus saline: does it make a difference? Should we reevaluate our practice?". AANA J 71 (6): 449–53. PMID 15098532.
- ^ Beilin Y, Arnold I, Telfeyan C, Bernstein HH, Hossain S (2000). "Quality of analgesia when air versus saline is used for identification of the epidural space in the parturient". Reg Anesth Pain Med. 25 (6): 596–9. doi:10.1053/rapm.2000.9535. PMID 11097666.
- ^ Rapp HJ, Folger A, Grau T (August 2005). "Ultrasound-guided epidural catheter insertion in children". Anesth Analg. 101 (2): 333–9, table of contents. doi:10.1213/01.ANE.0000156579.11254.D1. PMID 16037140.
- ^ Beilin Y, Bernstein HH, Zucker-Pinchoff B (August 1995). "The optimal distance that a multiorifice epidural catheter should be threaded into the epidural space". Anesth Analg. 81 (2): 301–4. doi:10.1097/00000539-199508000-00016. PMID 7618719. http://www.anesthesia-analgesia.org/cgi/pmidlookup?view=long&pmid=7618719.
- ^ Lim Y, Sia AT, Ocampo C (October 2005). "Automated regular boluses for epidural analgesia: a comparison with continuous infusion". Int J Obstet Anesth. 14 (4): 305–9. doi:10.1016/j.ijoa.2005.05.004. PMID 16154735.
- ^ Wong CA, Ratliff JT, Sullivan JT, Scavone BM, Toledo P, McCarthy RJ (March 2006). "A randomized comparison of programmed intermittent epidural bolus with continuous epidural infusion for labor analgesia". Anesth Analg. 102 (3): 904–9. doi:10.1213/01.ane.0000197778.57615.1a. PMID 16492849.
- ^ Sia AT, Lim Y, Ocampo C (March 2007). "A comparison of a basal infusion with automated mandatory boluses in parturient-controlled epidural analgesia during labor". Anesth Analg. 104 (3): 673–8. doi:10.1213/01.ane.0000253236.89376.60. PMID 17312228.
- ^ Basse L, Werner M, Kehlet H (2000). "Is urinary drainage necessary during continuous epidural analgesia after colonic resection?". Reg Anesth Pain Med. 25 (5): 498–501. doi:10.1053/rapm.2000.9537. PMID 11009235.
- ^ Kost-Byerly S, Tobin JR, Greenberg RS, Billett C, Zahurak M, Yaster M (April 1998). "Bacterial colonization and infection rate of continuous epidural catheters in children". Anesth Analg. 86 (4): 712–6. doi:10.1097/00000539-199804000-00007. PMID 9539589. http://www.anesthesia-analgesia.org/cgi/pmidlookup?view=long&pmid=9539589.
- ^ Kostopanagiotou G, Kyroudi S, Panidis D, et al. (2002). "Epidural catheter colonization is not associated with infection". Surgical Infections 3 (4): 359–65. doi:10.1089/109629602762539571. PMID 12697082.
- ^ Yuan HB, Zuo Z, Yu KW, Lin WM, Lee HC, Chan KH (January 2008). "Bacterial colonization of epidural catheters used for short-term postoperative analgesia: microbiological examination and risk factor analysis". Anesthesiology 108 (1): 130–7. doi:10.1097/01.anes.0000296066.79547.f3. PMID 18156891.
- ^ Kinirons B, Mimoz O, Lafendi L, Naas T, Meunier J, Nordmann P (February 2001). "Chlorhexidine versus povidone iodine in preventing colonization of continuous epidural catheters in children: a randomized, controlled trial". Anesthesiology 94 (2): 239–44. doi:10.1097/00000542-200102000-00012. PMID 11176087. http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?issn=0003-3022&volume=94&issue=2&spage=239.
- ^ Aram L, Krane EJ, Kozloski LJ, Yaster M (June 2001). "Tunneled epidural catheters for prolonged analgesia in pediatric patients". Anesth Analg. 92 (6): 1432–8. doi:10.1097/00000539-200106000-00016. PMID 11375820. http://www.anesthesia-analgesia.org/cgi/pmidlookup?view=long&pmid=11375820.
- ^ Bubeck J, Boos K, Krause H, Thies KC (September 2004). "Subcutaneous tunneling of caudal catheters reduces the rate of bacterial colonization to that of lumbar epidural catheters". Anesth Analg. 99 (3): 689–93, table of contents. doi:10.1213/01.ANE.0000130023.48259.FB. PMID 15333395.
- ^ Nitescu P, Sjöberg M, Appelgren L, Curelaru I (March 1995). "Complications of intrathecal opioids and bupivacaine in the treatment of "refractory" cancer pain". Clin J Pain. 11 (1): 45–62. doi:10.1097/00002508-199503000-00006. PMID 7540439.
- ^ Block BM, Liu SS, Rowlingson AJ, Cowan AR, Cowan JA, Wu CL (November 2003). "Efficacy of postoperative epidural analgesia: a meta-analysis". JAMA 290 (18): 2455–63. doi:10.1001/jama.290.18.2455. PMID 14612482.
- ^ Ballantyne JC, Carr DB, deFerranti S, et al. (March 1998). "The comparative effects of postoperative analgesic therapies on pulmonary outcome: cumulative meta-analyses of randomized, controlled trials". Anesth Analg. 86 (3): 598–612. doi:10.1097/00000539-199803000-00032. PMID 9495424. http://www.anesthesia-analgesia.org/cgi/pmidlookup?view=long&pmid=9495424.
- ^ a b c d Wilson IH, Allman KG (2006). Oxford handbook of anaesthesia. Oxford: Oxford University Press. p. 1038. ISBN 0-19-856609-3.
- ^ Beattie WS, Badner NH, Choi P (October 2001). "Epidural analgesia reduces postoperative myocardial infarction: a meta-analysis". Anesth Analg. 93 (4): 853–8. doi:10.1097/00000539-200110000-00010. PMID 11574345. http://www.anesthesia-analgesia.org/cgi/pmidlookup?view=long&pmid=11574345.
- ^ Yokoyama M, Itano Y, Katayama H, et al. (November 2005). "The effects of continuous epidural anesthesia and analgesia on stress response and immune function in patients undergoing radical esophagectomy". Anesth Analg. 101 (5): 1521–7. doi:10.1213/01.ANE.0000184287.15086.1E. PMID 16244024.
- ^ a b Gendall KA, Kennedy RR, Watson AJ, Frizelle FA (September 2007). "The effect of epidural analgesia on postoperative outcome after colorectal surgery". Colorectal Dis. 9 (7): 584–98; discussion 598–600. doi:10.1111/j.1463-1318.2007.1274.x. PMID 17506795.
- ^ Rigg JR, Jamrozik K, Myles PS, et al. (April 2002). "Epidural anaesthesia and analgesia and outcome of major surgery: a randomised trial". Lancet 359 (9314): 1276–82. doi:10.1016/S0140-6736(02)08266-1. PMID 11965272.
- ^ Krane EJ, Tyler DC, Jacobson LE (July 1989). "The dose response of caudal morphine in children". Anesthesiology 71 (1): 48–52. doi:10.1097/00000542-198907000-00009. PMID 2751139.
- ^ Jacobson L, Chabal C, Brody MC (November 1988). "A dose-response study of intrathecal morphine: efficacy, duration, optimal dose, and side effects". Anesth Analg. 67 (11): 1082–8. PMID 3189898.
- ^ Wüst HJ, Bromage PR (April 1987). "Delayed respiratory arrest after epidural hydromorphone". Anaesthesia 42 (4): 404–6. doi:10.1111/j.1365-2044.1987.tb03982.x. PMID 2438964.
- ^ Haberkern CM, Lynn AM, Geiduschek JM, et al. (December 1996). "Epidural and intravenous bolus morphine for postoperative analgesia in infants". Can J Anaesth. 43 (12): 1203–10. doi:10.1007/BF03013425. PMID 8955967.
- ^ a b Mayo Clinic Staff. "Labor and Delivery: Pain Medications - Epidural Block". switchDiv('2'); #content_2. http://www.mayoclinic.com/health/labor-and-delivery/PR00105. Retrieved July 5, 2011.
- ^ (Lassen K, Soop M, Nygren J, Cox PB, Hendry PO, Spies C, et al. Consensus review of optimal perioperative care in colorectal surgery. Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg 2009;144:961–9. )
- ^ (Agaram R, Douglas MJ, McTaggart RA, Gunka V. Inadequate pain relief with labor epidurals: a multivariate analysis of associated factors. Int J Obstet Anesth. 2009.18(1):10-4.)
- ^ Norris MC, Leighton BL, DeSimone CA (May 1989). "Needle bevel direction and headache after inadvertent dural puncture". Anesthesiology 70 (5): 729–31. doi:10.1097/00000542-198905000-00002. PMID 2655500.
- ^ Sprigge JS, Harper SJ (January 2008). "Accidental dural puncture and post dural puncture headache in obstetric anaesthesia: presentation and management: a 23-year survey in a district general hospital". Anaesthesia 63 (1): 36–43. doi:10.1111/j.1365-2044.2007.05285.x. PMID 18086069.
- ^ a b Wilson IH, Allman KG (2006). Oxford handbook of anaesthesia. Oxford: Oxford University Press. p. 20. ISBN 0-19-856609-3.
- ^ YF Wang; SJ Wang. "Headache associated with low CSF pressure". Medlink-Neurology Summary. http://www.medlink.com/web_content/index/MLT002PE.asp.
- ^ Jonas W, Johansson LM, Nissen E et al. (2009). "Effects of Intrapartum Oxytocin Administration and Epidural Analgesia on the Concentration of Plasma Oxytocin and Prolactin, in Response to Suckling During the Second Day Postpartum". Breastfeed Med. 4 (2): 71–82. doi:10.1089/bfm.2008.0002. PMID 19210132.
- ^ Clarkson CW, Hondeghem LM (April 1985). "Mechanism for bupivacaine depression of cardiac conduction: fast block of sodium channels during the action potential with slow recovery from block during diastole". Anesthesiology 62 (4): 396–405. doi:10.1097/00000542-198504000-00006. PMID 2580463.
- ^ Groban L, Deal DD, Vernon JC, James RL, Butterworth J (January 2001). "Cardiac resuscitation after incremental overdosage with lidocaine, bupivacaine, levobupivacaine, and ropivacaine in anesthetized dogs". Anesth Analg. 92 (1): 37–43. doi:10.1097/00000539-200101000-00008. PMID 11133597. http://www.anesthesia-analgesia.org/cgi/pmidlookup?view=long&pmid=11133597.
- ^ a b c d "Epidurals and risk: it all depends [May 2007; 159-3"]. http://www.medicine.ox.ac.uk/bandolier/band159/b159-3.html.
- ^ a b Wilson IH, Allman KG (2006). Oxford handbook of anaesthesia. Oxford: Oxford University Press. p. 21. ISBN 0-19-856609-3.
- ^ Rice I, Wee MY, Thomson K (January 2004). "Obstetric epidurals and chronic adhesive arachnoiditis". Br J Anaesth. 92 (1): 109–20. doi:10.1093/bja/aeh009. PMID 14665562. http://bja.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=14665562.
- ^ Wilson IH, Allman KG (2006). Oxford handbook of anaesthesia. Oxford: Oxford University Press. p. 1039. ISBN 0-19-856609-3.
- ^ Whitehead, Saffron A.; Nussey, Stephen (2001). Endocrinology: an integrated approach. Oxford: BIOS. pp. 122. ISBN 1-85996-252-1.
- ^ Gregory, Michael Ph.D. "Endocrine System: Posterior Pituitary". http://faculty.clintoncc.suny.edu/faculty/michael.gregory/files/bio%20102/bio%20102%20lectures/endocrine%20system/endocrin.htm#Posterior%20pituitary. Retrieved 5 July 2011.
- ^ Takayanagi Y, Yoshida M, Bielsky IF, et al. (November 2005). "Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice". Proceedings of the National Academy of Sciences of the United States of America 102 (44): 16096–101. doi:10.1073/pnas.0505312102. PMC 1276060. PMID 16249339. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1276060.
- ^ Seyb ST, Berka RJ, Socol ML, Dooley SL (1999). "Risk of cesarean delivery with elective induction of labor at term in nulliparous women". Obstet Gynecol 94 (4): 600–607. doi:10.1016/S0029-7844(99)00377-4. PMID 10511367.
- ^ Liu EHC, Sia ATH (2004). "Rates of caesarean section and instrumental vaginal delivery in nulliparous women after low concentration epidural infusions or opioid analgesia: systematic review". BMJ 328 (7453): 1410–1415. doi:10.1136/bmj.38097.590810.7C.
- ^ Halpern SH, Muir H, Breen TW et al. (2004). "A multicenter randomized controlled trial comparing patient-controlled epidural with intravenous analgesia for pain relief in labor". Anesth Analg 99 (5): 1532–1538. doi:10.1213/01.ANE.0000136850.08972.07. PMID 15502060.
- ^ Wong CA, Scavone BM, Peaceman AM et al. (2005). "The risk of cesarean delivery with neuraxial analgesia given early versus late in labor". N Engl J Med 352 (7): 655–665. doi:10.1056/NEJMoa042573. PMID 15716559.
- ^ Ohel G, Gonen R, Vaida S, Barak S, Gaitini L (2006). "Early versus late initiation of epidural analgesia in labor: does it increase the risk of cesarean section? A randomized trial". Am J Obstet Gynecol 194 (3): 600–605. doi:10.1016/j.ajog.2005.10.821. PMID 16522386.
- ^ Wong CA, McCarthy RJ, Sullivan JT, Scavone BM, Gerber SE, Yaghmour EA. Early compared with late neuraxial analgesia in nulliparous labor induction: a randomized controlled trial.
- ^ a b "Epidural versus non-epidural analgesia in labour pain". http://www.jr2.ox.ac.uk/bandolier/booth/painpag/Acutrev/labour/AP056.html.
- ^ Patel SS, Dunn CJ, Bryson HM (1996). "Epidural clonidine: a review of its pharmacology and efficacy in the management of pain during labour and postoperative and intractable pain". CNS Drugs 6 (6): 474–497.
- ^ Anim-Somuah M, Smyth R, Howell C (2005). Anim-Somuah, Millicent. ed. "Epidural versus non-epidural or no analgesia in labour". Cochrane Database Syst Rev.(Online) (4): CD000331. doi:10.1002/14651858.CD000331.pub2. PMID 16235275.
- ^ Klein Michael C., MD (April 2006). "Does epidural analgesia increase rate of cesarean section?". Can Fam Physician 52 (4): 419–421. PMC 1481670. PMID 16639961. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1481670.
- ^ Comparative Obstetric Mobile Epidural Trial (COMET) Study Group UK (July 2001). "Effect of low-dose mobile versus traditional epidural techniques on mode of delivery: a randomised controlled trial". Lancet 358 (9275): 19–23. doi:10.1016/S0140-6736(00)05251-X. PMID 11454372.
- ^ Thorp JA, Breedlove G (June 1996). "Epidural analgesia in labor: an evaluation of risks and benefits". Birth 23 (2): 63–83. doi:10.1111/j.1523-536X.1996.tb00833.x. PMID 8826170.
- ^ Torvaldsen S, Roberts CL, Simpson JM, Thompson JF, Ellwood DA (2006). "Intrapartum epidural analgesia and breastfeeding: a prospective cohort study". Int Breastfeed J. 1: 24. doi:10.1186/1746-4358-1-24. PMC 1702531. PMID 17134489. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1702531.
- ^ Camann W (July 2007). "Labor analgesia and breast feeding: avoid parenteral narcotics and provide lactation support". Int J Obstet Anesth. 16 (3): 199–201. doi:10.1016/j.ijoa.2007.03.008. PMID 17521903.
- ^ a b J. C. Diz, A. Franco, D. R. Bacon, J. Rupreht, and J. Alvarez (eds.); The history of anesthesia: proceedings of the Fifth International Symposium, Elsevier (2002), pp. 205-6, 0-444-51003-6
- ^ a b "Robert A. Hingson, et al." Current Biography 1943, pp300-04
- ^ Current Biography 1943, p301
- ^ "Childbirth Made Painless and Safe By New Methods," AP article by Willis Young, reprinted in Oakland Tribune, January 21, 1943
Further reading
- Roberts CL, Tracy S, Peat B (July 2000). "Rates for obstetric intervention among private and public patients in Australia: population based descriptive study". BMJ 321 (7254): 137–41. doi:10.1136/bmj.321.7254.137. PMC 27430. PMID 10894690. http://bmj.com/cgi/pmidlookup?view=long&pmid=10894690.
- Zhang J, Yancey MK, Klebanoff MA, Schwarz J, Schweitzer D (July 2001). "Does epidural analgesia prolong labor and increase risk of cesarean delivery? A natural experiment". Am J Obstet Gynecol. 185 (1): 128–34. doi:10.1067/mob.2001.113874. PMID 11483916. http://www.sciencedirect.com/science/article/B6W9P-45V21G6-S/2/f04efd0d9a6ec5c61b5685187fa09836.
- Leighton BL, Halpern SH (May 2002). "The effects of epidural analgesia on labor, maternal, and neonatal outcomes: a systematic review". Am J Obstet Gynecol. 186 (5 Suppl Nature): S69–77. PMID 12011873. http://linkinghub.elsevier.com/retrieve/pii/a121813.
- Boqing Chen and Patrick M. Foye, UMDNJ: New Jersey Medical School, Epidural Steroid Injections: Non-surgical Treatment of Spine Pain, eMedicine: Physical Medicine and Rehabilitation (PM&R), August 2005. Also available online.
External links
- Epidurals for pain relief in labour Comprehensive information with women's stories - informedhealthonline.org, Accessed July 2, 2009.
- Epidural and nerve root injections pain treatment explained
- What Is An Epidural Headache? - Epidural Headaches Explained
Routes of administration / Dosage forms Oral Buccal / Sublabial / Sublingual- Mouthwash
- Toothpaste
- Ointment
- Oral spray
- Oxygen mask
- Oxygen concentrator
- Anaesthetic machine
- Relative analgesia machine





Ocular / Otologic / Nasal - Nasal spray
- Ear drops
- Eye drops
- Ointment
- Hydrogel
- Nanosphere suspension
- Mucoadhesive microdisc (microsphere tablet)
Urogenital - Ointment
- Pessary (vaginal suppository)
- Vaginal ring
- Vaginal douche
- Intrauterine device (IUD)
- Extra-amniotic infusion
- Intravesical infusion
Rectal (enteral) - Ointment
- Suppository
- Enema (Solution • Hydrogel)
- Murphy drip
- Nutrient enema
Dermal Injection / Infusion
(into tissue/blood)- Intracavernous
- Intravitreal
- Intra-articular or intrasynovial injection
- Transscleral
- Intracerebral
- Intrathecal
- Epidural
Additional explanation: Mucous membranes are used by the human body to absorb the dosage for all routes of administration, except for "Dermal" and "Injection/Infusion".
Administration routes can also be grouped as Topical (local effect) or Systemic (defined as Enteral = Digestive tract/Rectal, or Parenteral = All other routes).Routes of administration by organ system Gastrointestinal Respiratory system Pulmonary • NasalVisual system / Auditory system Ocular (Ocular-topical / Intravitreal / Transscleral) • Otologic (Oto-topical)Reproductive system Intracavernous • Intravaginal • Intrauterine (Extra-amniotic)Urinary system IntravesicalPeritoneum Central nervous system Intracerebral • Intrathecal • EpiduralCirculatory system Musculoskeletal system Skin Epicutaneous • Intradermal • SubcutaneousCategories:- Childbirth
- Regional anesthesia
- Obstetrical procedures
- Dosage forms
Wikimedia Foundation. 2010.



