- Cyclobutane
-
Cyclobutane 

 cyclobutane
cyclobutaneIdentifiers CAS number 00287-23-0 
Jmol-3D images Image 1 - C1CCC1
Properties Molecular formula C4H8 Molar mass 56.107 g/mol Density 0.720 g/cm3 Melting point -91 °C, 182 K, -132 °F
Boiling point 12.5 °C, 286 K, 55 °F
Related compounds Related alkane Butane Related compounds Cyclobutene; Cyclobutadiene  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Cyclobutane is an organic compound with the formula (CH2)4. Cyclobutane is a colourless gas and commercially available as a liquefied gas. Derivatives of cyclobutane are called cyclobutanes. Cyclobutane itself is of no commercial or biological significance, but more complex derivatives are important in biology and biotechnology.
Contents
Structure
The bond angles between carbon atoms are significantly strained and as such have lower bond energies than related linear or unstrained hydrocarbons, e.g. butane or cyclohexane. As such, cyclobutane is unstable above about 500 °C.
The four carbon atoms in cyclobutane are not coplanar, instead the ring typically adopts a folded or "puckered" conformation.[1] One of the carbon atoms makes a 25° angle with the plane formed by the other three carbons. In this way some of the eclipsing interactions are reduced. The conformation is also known as a "butterfly". Equivalent puckered conformations interconvert:
Cyclobutanes in biology and biotechnology
Despite inherent strain the cyclobutane motif is indeed found in nature. One unusual example is pentacycloanammoxic acid,[2] which is a ladderane composed of 5 fused cyclobutane units. The estimated strain in this compound is 3 times that of cyclobutane. The compound is found in bacteria performing the anammoxprocess where it forms part of a tight and very dense membrane believed to protect the organism from toxic hydroxylamine and hydrazine involved in the production of nitrogen and water from nitrite ions and ammonia.[3] Some related fenestranes are also found in nature.[citation needed]
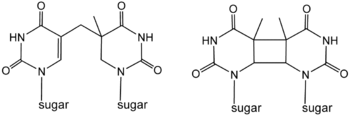
Cyclobutane photodimers ("CPD's") are formed by photochemical reactions that result in the coupling of the C=C double bonds of pyrimidines.[4][5][6] T-T dimers thymine dimers formed in between two thymines are the most abundant of the CPD's. CPD's are readily repaired by nucleotide excision repair enzymes. In most organisms they can also be repaired by photolyases, a light-dependent family of enzymes. Xeroderma pigmentosum is a genetic disease where this damage can not be repaired, resulting in skin discolouration and tumours induced by exposure to UV light.
Carboplatin is a popular anticancer drug that is derived from cyclobutane-1,1-carboxylic acid.
Preparation
Many methods exist for the preparation of cyclobutanes. Alkenes dimerize upon irradiation with UV-light. 1,4-Dihalobutanes convert to cyclobutanes upon dehalogenation with reducing metals.
See also
References
- ^ chemical compound :: Cycloalkanes - Britannica Online Encyclopedia
- ^ J. S. Sinninghe Damsté, M. Strous, W. I. C. Rijpstra, E. C. Hopmans, J. A. J. Geenevasen, A. C. T. van Duin, L. A. van Niftrik and M. S. M. Jetten (2002). "Linearly concatenated cyclobutane lipids form a dense bacterial membrane". Nature 419 (6908): 708–712. doi:10.1038/nature01128. PMID 12384695.
- ^ Vincent Mascitti and E. J. Corey (2006). "Enantioselective Synthesis of Pentacycloanammoxic Acid". J. Am. Chem. Soc. 128 (10): 3118–9. doi:10.1021/ja058370g. PMID 16522072.Authors state that mode of biosynthesis is quite mysterious
- ^ R. B. Setlow (1966). "Cyclobutane-Type Pyrimidine Dimers in Polynucleotides". Science 153 (3734): 379–386. doi:10.1126/science.153.3734.379. PMID 5328566.
- ^ Expert reviews in molecular medicine (2 December 2002). "Structure of the major UV-induced photoproducts in DNA.". Cambridge University Press. http://www-ermm.cbcu.cam.ac.uk/02005331a.pdf.
- ^ Christopher Mathews and K.E. Van Holde (1990). Biochemistry (2nd ed.). Benjamin Cummings Publication. p. 1168. ISBN 978-0805350159.
External links
- Datasheet Link
Cyclopropane · Cyclobutane · Cyclopentane · Cyclohexane · Cycloheptane · Cyclooctane · Cyclononane · Cyclodecane · Cycloundecane · CyclododecaneCategories:- Cycloalkanes
Wikimedia Foundation. 2010.