- Perfluorooctanoic acid
-
Perfluorooctanoic acid  pentadecafluorooctanoic acidOther namesperfluorooctanoic acid, PFOA, C8, perfluorooctanoate, perfluorocaprylic acid, FC-143, F-n-octanoic acid, PFO
pentadecafluorooctanoic acidOther namesperfluorooctanoic acid, PFOA, C8, perfluorooctanoate, perfluorocaprylic acid, FC-143, F-n-octanoic acid, PFOIdentifiers CAS number 335-67-1 
PubChem 9554 ChemSpider 9180 
EC number 206-397-9 ChEBI CHEBI:35549 
ChEMBL CHEMBL172988 
RTECS number RH0781000 Jmol-3D images Image 1 - FC(F)(C(F)(F)C(=O)O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F
Properties Molecular formula C8HF15O2 Molar mass 414.07 g/mol Appearance colorless liquid Density 1.8 g/cm3[1] Melting point 40–50 °C[1]
Boiling point 189–192 °C[1]
Solubility in water soluble, 9.5 g/L (PFO)[2] Solubility in other solvents polar organic
solventsAcidity (pKa) ~0[3][4][5] Hazards MSDS [1] R-phrases R22 R34 R52/53 S-phrases S26 S36/37/39 S45 Main hazards Strong Acid, Causes Burns Related compounds Related compounds Perfluorooctanesulfonic acid (PFOS), Perfluorononanoic acid (PFNA), Perfluorooctanesulfonamide (PFOSA), Trifluoroacetic acid (TFA)  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Perfluorooctanoic acid (PFOA), also known as C8 and perfluorooctanoate, is a synthetic, stable perfluorinated carboxylic acid and fluorosurfactant. One industrial application is as a surfactant in the emulsion polymerization of fluoropolymers. It has been used in the manufacture of such prominent consumer goods as Teflon and GoreTex. PFOA has been manufactured since the 1940s in industrial quantities. It is also formed by the degradation of precursors such as some fluorotelomers.
PFOA persists indefinitely in the environment. It is a toxicant and carcinogen in animals. PFOA has been detected in the blood of more than 98% of the general US population in the low and sub-parts per billion range, and levels are higher in chemical plant employees and surrounding subpopulations. Exposure has been associated with increased cholesterol and uric acid levels, and recently higher serum levels of PFOA were found to be associated with increased risk of chronic kidney disease in the general United States population, consistent with earlier animal studies.[6] "This association was independent of confounders such as age, sex, race/ethnicity, body mass index, diabetes, hypertension, and serum cholesterol level."[6]
As a result of a class-action lawsuit and community settlement with DuPont, three epidemiologists are conducting studies on the population surrounding a chemical plant that was exposed to PFOA at levels greater than in the general population. If PFOA exposure is found to be likely to lead to an increased risk of disease, future liabilities for DuPont will be triggered. Full results from the studies are expected in 2012.
How general populations are exposed to PFOA is not completely understood. PFOA has been detected in industrial waste, stain resistant carpets, carpet cleaning liquids, house dust, microwave popcorn bags, water, food, and PTFE. Although some cookware is marketed as PFOA-free, PTFE non-stick cookware is considered an insignificant exposure pathway.
Contents
History
In 1947, 3M began producing PFOA by electrochemical fluorination.[2] In 1951, DuPont started using PFOA in the manufacturing of fluoropolymers in Washington, WV.[7] In 1961, DuPont was aware of hepatomegaly in mice fed with PFOA.[8][9] In 1968, organofluorine content was detected in the blood serum of consumers, and in 1976 it was suggested to be PFOA or a related compound such as PFOS.[10][11][12] In the 1980s and 1990s researchers investigated the toxicity of PFOA.[9]
In 1999, the United States Environmental Protection Agency (USEPA) began investigating perfluorinated chemicals after receiving data on the global distribution and toxicity of PFOS.[13] For these reasons, and USEPA pressure,[14] in May 2000, 3M announced the phaseout of the production of PFOA, PFOS, and PFOS-related products.[15] 3M stated that they would have made the same decision regardless of USEPA pressure.[16]
Because of the 3M phaseout, in 2002 DuPont built its own plant in Fayetteville, NC to manufacture the chemical.[17] The chemical has received attention due to litigation from the PFOA-contaminated community around DuPont's Washington Works Washington, WV facility, along with USEPA focus. Research on PFOA has demonstrated ubiquity, animal-based toxicity, and some associations with human health parameters and potential health effects. Additionally, advances in analytical chemistry in recent years have allowed the routine detection of low- and sub-parts per billion levels of PFOA in a variety of substances.[12]
Synthesis
PFOA has two main synthesis routes, electrochemical fluorination (ECF) and telomerization.[2] The equation below represents the ECF route with hydrofluoric acid reacting with octanoic acid chloride.[18]
- H(CH2)7COCl + 17 HF → H(CH2)7COF + C7H16 + 2 C8F16O + HCl + H2
The equation above shows the multiple products of ECF. The target product, F(CF2)7COF (not represented) is produced as only 10–15% of the total product, while the main products are perfluorinated cyclic ether isomers, including FC-75.[18] To yield PFOA, the perfluorinated acid fluoride is hydrolyzed. The PFOA formed by this method is a mixture of straight chain (78%), terminally branched (13%), and internally branched (9%) molecules, as ECF rearranges the carbon "tail" of the acid chloride.[18] ECF also results in production wastes.[19] 3M synthesized ECF PFOA at their Cottage Grove, MN facility from 1947 to 2002 and was the world's largest producer.[2][19] ECF production continues on a smaller scale in Europe and Asia.[2]
PFOA is also synthesized by the telomerization represented below, where the telogen is the organoiodine compound and the taxogen is the unsaturated tetrafluoroethylene.[18][20]
- C2F5I + 3 C2F4 → C2F5(C2F4)3I
The product is oxidized by SO3 to form PFOA.[18] Under reaction conditions, telomers form with varying length chains containing an even number of carbon atoms, as products mostly contain two to six tetrafluoroethylene taxogens.[18] After oxidation, distillation is used to separate PFOA from the other perfluorinated carboxylic acids.[18] The telomerization synthesis of PFOA was pioneered by DuPont,[18] and it is not well suited to the laboratory.[20] PFOA formed by telomerization is completely linear, in contrast to the mixture of structures formed by ECF.
Applications
PFOA has widespread applications. In 1976 PFOA was reported as a water and oil repellent "in fabrics and leather and in the production of floor waxes and waxed papers";[21] however, it is believed that paper is no longer treated with perfluorinated compounds, but with fluorotelomers with less than 0.1% PFOA.[22] The compound is also used in "insulators for electric wires, planar etching of fused silica",[20] fire fighting foam,[2][23] and outdoor clothing.[24] As a protonated species, the acid form of PFOA was the most widely used perfluorocarboxylic acid used as a reactive intermediate in the production of fluoroacrylic esters.[25][26]
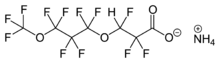 ADONA, ammonium 4,8-dioxa-3H-perfluorononanoate, is 3M's PFOA replacement in the emulsion polymerization of fluoropolymers.[27]
ADONA, ammonium 4,8-dioxa-3H-perfluorononanoate, is 3M's PFOA replacement in the emulsion polymerization of fluoropolymers.[27]
As a salt, the dominant use is as an emulsifier for the emulsion polymerization of fluoropolymers such as polytetrafluoroethylene (PTFE, or Teflon), polyvinylidene fluoride, and fluoroelastomers.[28][29] For this use, 3M subsidiary Dyneon has a replacement emulsifer[30] despite DuPont stating PFOA is an "essential processing aid".[31] PFOA is used in the production of Gore-Tex[32] as it is PTFE-based. In PTFE processing, PFOA is in aqueous solution and forms micelles that contain tetrafluoroethylene and the growing polymer.[33] PFOA can be used to stabilize fluoropolymer and fluoroelastomer suspensions prior to further industrial processing and in ion-pair reversed-phase liquid chromatography it can act as an extraction agent.[34] PFOA also finds uses in electronic products and as an industrial fluorosurfactant.[32][35]
In a 2009 USEPA study of 116 products—purchased between March 2007 and May 2008 and found to contain at least 0.01% fluorine by weight—the concentrations of PFOA were determined.[36] Concentrations shown below range from not detected, or ND, (with the detection limit in parenthesis) to 6750 with concentrations in nanograms of PFOA per gram of sample—or parts per billion—unless stated otherwise.
Product Range Pre-treated carpeting ND (<1.5) to 462 Carpet-care liquids 19 to 6750 Treated apparel 5.4 to 161 Treated upholstery 0.6 to 293 Treated home textiles 3.8 to 438 Treated non-woven medical garments 46 to 369 Industrial floor wax and wax removers 7.5 to 44.8 Stone, tile, and wood sealants 477 to 3720 Membranes for apparel 0.1 to 2.5 ng/cm2 Food contact paper ND (<1.5) to 4640 Dental floss/tape ND (<1.5) to 96.7 Thread sealant tape ND (<1.5) to 3490 PTFE cookware ND (<1.5) to 4.3 Properties
The carboxylate "head" of PFOA is hydrophilic while the fluorocarbon tail is hydrophobic and lipophobic. The "tail" is hydrophobic due to being non-polar and lipophobic because fluorocarbons are less susceptible to the London dispersion force than hydrocarbons.[37] PFOA is an ideal surfactant because it can lower the surface tension of water more than hydrocarbon surfactants while possessing exceptional stability due to the presence of multiple carbon–fluorine bonds.[35][37] The stability of PFOA is desired industrially, but a cause of concern environmentally. PFOA is resistant to degradation by natural processes such as metabolism, hydrolysis, photolysis, or biodegradation[25] making it persist indefinitely[38] in the environment.
PFOA is found in environmental and biological fluids as the anion perfluorooctanoate.[39] PFOA is absorbed from ingestion and can penetrate skin.[10] The oxygens on PFOA are how it binds proteins with fatty acid or hormone substrates such as serum albumin, liver fatty acid-binding protein, and the nuclear receptors PPARα[29] and possibly CAR.[40] In animals, PFOA is mainly present in the liver, blood, and kidneys.[10] PFOA does not accumulate in fat tissue, unlike traditional organohalogen persistent organic pollutants.[41] In humans, PFOA has an average elimination half-life of about 3 years.[42][43][44] Because of this long half-life,[45] PFOA has the potential to bioaccumulate.
Global occurrence and sources
PFOA contaminates every continent.[46] PFOA has been detected in the central Pacific Ocean at low parts per quadrillion ranges, and at low parts per trillion levels in coastal waters.[47] Due to the surfactant nature of PFOA, it has been found to concentrate in the top layers of ocean water.[48] PFOA is detected widely in surface waters, and is present in numerous mammals, fish, and bird species.[46] However, wildlife has much less PFOA than humans, unlike PFOS[49] and other longer perfluorinated carboxylic acids;[50] in wildlife, PFOA is not as bioaccumulative as longer perfluorinated carboxylic acids.[41]
Most industrialized nations have average PFOA blood serum levels ranging from 2 to 8 parts per billion;[51] the highest consumer sub-population identified was in Korea—with about 60 parts per billion.[49] In Peru,[52] Vietnam,[53] and Afghanistan[54] blood serum levels have been recorded to be below one part per billion. In 2003–2004 99.7% of Americans had detectable PFOA in their serum with an average of about 4 parts per billion,[55] and concentrations of PFOA in US serum have declined by 25% in recent years.[56] Despite a decrease in PFOA, the longer perfluorinated carboxylic acid PFNA is increasing in the blood of US consumers.[55]
Industrial sources
PFOA is released directly from industrial sites. For example, the DuPont Washington Works facility in Washington, WV estimated total PFOA emissions of 80,000 pounds (lbs) in 2000 and 1,700 pounds in 2004.[7] A 2006 study, with two of four authors DuPont employees, estimated about 80% of historical perfluorocarboxylate emissions were released to the environment from fluoropolymer manufacture and use.[2] PFOA can be measured in water from industrial sites other than fluorochemical plants. PFOA has also been detected in emissions from the carpet,[57] paper[58] and electronics industries.[59] The most important emission sources are carpet and textile protection as well as fire-fighting foams.[60]
Precursors
PFOA can form as a breakdown product from a variety of precursor molecules. PFOA precursors can be transformed to PFOA by metabolism, biodegradation, or atmospheric processes. Examples include 8:2 fluorotelomer alcohol (F(CF2)8CH2CH2OH), polyfluoroalkyl phosphate surfactants (PAPS),[61] and possibly N-EtFOSE alcohol (F(CF2)8SO2N(Et)CH2CH2OH).[46][62] When PTFE is degraded by heat (pyrolysis) it can form PFOA as a minor product.[63][64] The Organisation for Economic Co-operation and Development (OECD) has compiled a list of 615 chemicals that have the potential to break down into perfluorocarboxylic acids (PFCA) including PFOA.[65] However, not all 615 have the potential to break down to form PFOA.
A majority of waste water treatment plants (WWTPs) that have been tested output more PFOA than is input, and this increased output has been attributed to the biodegradation of fluorotelomer alcohols.[66] A current PFOA precursor concern are fluorotelomer-based polymers; fluorotelomer alcohols attached to hydrocarbon backbones via ester linkages may detach and be free to biodegrade to PFOA.[67]
Sources to people
Food,[68] drinking water, outdoor air, indoor air,[69] dust, and food packagings[70] are all implicated as sources of PFOA to people.[61] However, it is unclear which exposure routes dominate[38] because of data gaps. When water is a source, blood levels are approximately 100 times higher than drinking water levels.[71][72]
Citizens that lived in the PFOA contaminated area around DuPont's Washington Works Washington, WV facility were found to have higher levels of PFOA in their blood from drinking water. The highest PFOA levels in drinking water were found in the Little Hocking water system, with an average concentration of 3.55 parts per billion during 2002–2005.[7] Individuals who drank more tap water, ate locally grown fruits and vegetables, or ate local meat, were all associated with having higher PFOA levels. Residents who used water carbon filter systems had lower PFOA levels.
Food contact surfaces
PFOA is also formed as an unintended byproduct in the production of fluorotelomers[73] and is present in finished goods treated with fluorotelomers, including those intended for food contact. Fluorotelomers are applied to food contact papers because they are lipophobic: they prevent oil from soaking into the paper from fatty foods. Also, fluorotelomers can be metabolized into PFOA.[74] In an U.S. Food and Drug Administration (USFDA) study, lipophobic fluorotelomer-based paper coatings (which can be applied to food contact paper in the concentration range of 0.4%) were found to contain 88,000–160,000 parts per billion PFOA, while microwave popcorn bags contained 6–290 parts per billion PFOA.[75] Toxicologists estimate that microwave popcorn could account for about 20% of the PFOA levels measured in an individual consuming 10 bags a year if 1% of the fluorotelomers are metabolized to PFOA.[74] Fluorotelomer coatings are used in fast food wrappers, candy wrappers, and pizza box liners.[76] PAPS, a type of paper fluorotelomer coating, and PFOA precursor, is also used in food contact papers.[61]
Despite DuPont's asserting that "cookware coated with DuPont Teflon non-stick coatings does not contain PFOA,"[77] residual PFOA was also detected in finished PTFE products including PTFE cookware (4–75 parts per billion).[75] However, PFOA levels ranged from undetectable (<1.5) to 4.3 parts per billion in a more recent study.[36] Also, non-stick cookware is heated—which should volatilize PFOA; PTFE products that are not heated, such as PTFE sealant tape, had higher (1800 parts per billion) levels detected.[78] Overall, PTFE cookware is considered an insignificant exposure pathway to PFOA.[79][80]
Potential path: sludge to food
PFOA and PFOS were detected in "very high" (low parts per million) levels in agricultural fields for grazing beef cattle[38] and crops[81] around Decatur, AL. The approximately 5000 acres of land were fertilized with "treated municipal sewage sludge, or biosolids."[38] PFOA was also detected in the blood of the cattle.[82] The water treatment plant received process wastewater from a nearby perfluorochemical manufacturing plant. 3M says they managed their own wastes, but Daikin America "discharged process wastewater to the municipal waste treatment plant."[38] If traced to meat, it would be the first time perfluorochemicals were traced from sludge to food.[38] However, the USDA reported—with a detection limits of 20 parts per billion—non-detectable levels for both PFOA and PFOS in cattle muscle tissue.[83]
Regulatory status
Drinking water and products
While there is no "legally enforceable federal standard"[81] for the level of PFOA in drinking water in the US, on January 15, 2009, the Bush administration U.S. Environmental Protection Agency[82] (USEPA) set a "provisional health advisory"[84] of 0.4 parts per billion in response to the detection of PFOA in agricultural soil. However, the advisory is not meant to protect the public from long term exposure but might protect individuals for "a couple of years."[81] While water companies are not required to test for PFOA, it is a potential candidate for regulation under the Safe Drinking Water Act.[81] As for consumer products, there is no federal safety standard for PFOA in the US.[85]
California and food packaging
An attempt to regulate PFOA in food packaging occurred in the US state of California in 2008. A bill, sponsored by State Senator Ellen Corbett and the Environmental Working Group, was approved that would have banned PFOA, PFOS, and related seven or more fluorinated carbon compounds in food packaging starting in 2010,[85][86] but the bill was vetoed by Governor Schwarzenegger.[87] The bill would have affected fluorochemical manufacturers outside of the state, and Schwarzenegger was lobbied by the chemical industry to veto. Schwarzenegger said the compound should be reviewed by the newly established, and more comprehensive, state program.[87]
Fluorotelomers
Main article: FluorotelomerFluorotelomer-based products are under extended investigation to determine their potential to degrade to PFOA; these studies could lead the USEPA to require DuPont and others to reformulate products with a value over $1 billion.[88]
Health concerns
Toxicology data
PFOA is a carcinogen, liver toxicant, a developmental toxicant, an immune system toxicant, and also exerts hormonal effects including alteration of thyroid hormone levels.[29] Animal studies show developmental toxicity from reduced birth size, physical developmental delays, endocrine disruption, and neonatal mortality.[46][89] PFOA causes liver cancer in rodents and also induces testicular and pancreatic cancer[90] through induction of Leydig cell tumors and pancreatic acinar cell tumors.[91] PFOA alters lipid metabolism.[46] It is an agonist of PPARα and is a peroxisome proliferator in rodents contributing to a well understood form of oxidative stress.[92] Humans are considered less susceptible to peroxisome proliferation than rodents. However, PFOA has been found to be a liver carcinogen in rainbow trout via a potential estrogenic mechanism, which may be more relevant to humans.[92] A study found PFOA to be an obesogen in female mice at mid-age—with altered levels of insulin and leptin—at the lowest dose of 0.01 milligrams per kilogram of body weight during development.[93]
While a USEPA review notes PFOA has not "been shown to be mutagenic in a variety of assays"[29] in 1991 researchers from Japan demonstrated oxidative liver DNA damage in an experiment with rats.[94] PFOA has been described as a member of a group of "classic non-genotoxic carcinogens".[95] However, a provisional German assessment notes that a 2005 study found PFOA to be genotoxic via a peroxisome proliferation pathway that produced oxygen radicals in HepG2 cells, and a 2006 study demonstrated the induction and suppression of a broad range of genes; therefore, it states that the indirect genotoxic (and thus carcinogenic) potential of PFOA cannot be dismissed.[96] Criteria have been proposed that would allow PFOA, and other perfluorinated compounds, to be classified as "weakly non-specific genotoxic."[97]
Human data
The levels of PFOA exposure in humans vary widely. While an average American might have 3 or 4 parts per billion of PFOA present in his blood serum,[98] individuals occupationally exposed to PFOA have had blood serum levels over 100,000 parts per billion (100 parts per million or 0.01%) recorded.[99] In a study of individuals living around DuPont's Washington Works WV plant, those who had no occupational exposure had a median blood serum level of 329 parts per billion while the median of those with occupational exposure was 775 parts per billion.[7] While no amount of PFOA in humans is legally recognized as harmful, DuPont was "not satisfied" with data showing their Chinese workers accumulated an average of about 2,250 parts per billion of PFOA in their blood from a starting average of around 50 parts per billion less than a year prior.[17]
Consumers
Single cross-sectional studies on consumers have been published noting multiple associations. Blood serum levels of PFOA were associated with an increased time to pregnancy—or "infertility"—in a 2009 study.[100] PFOA exposure was associated with decreased semen quality,[101] increased serum alanine aminotransferase levels,[102] and increased occurrence of thyroid disease.[45] In a study of 2003–2004 US samples, a higher (9.8 milligram per deciliter) total cholesterol level was observed when the highest quartile was compared to the lowest.[103] Along with other related compounds, PFOA exposure was associated with an increased risk of attention deficit hyperactivity disorder (ADHD) in a study of US children aged 12–15.[104] In a paper presented at the 2009 annual meeting of the International Society of Environmental Epidemiology,[105] PFOA appeared to act as an endocrine disruptor by a potential mechanism on breast maturation in young girls.[106] A C8 Science Panel status report noted an association between exposure in girls and a later onset of puberty.[107]
PFOA has been associated with signs of reduced fetal growth including lower birth weight.[108][109][110] However, other studies have not replicated the lower birth weight finding[111][112] including a study on the DuPont exposed community.[113] PFOA exposure in the Danish general population was not associated with an increased risk of prostate, bladder, pancreatic, or liver cancer.[114] Maternal PFOA levels were not associated with an offspring's increased risk of hospitalization due to infectious diseases,[115] behavioral and motor coordination problems,[116] or delays in reaching developmental milestones.[117]
Employees and DuPont exposed community
In 2010, the three members of the C8 Science Panel published a review of the epidemiological evidence on PFOA exposure in Environmental Health Perspectives.[43] Insufficient evidence exists to conclude PFOA causes adverse health effects in humans, but consistent evidence exists on associations with higher cholesterol and uric acid. Whether or not these potential effects result in an increase in cardiovascular disease is unknown.[118] Further data on the 69,030 member cohort[119] that is being studied by the panel is scheduled for release through 2012.[120]
Facial birth defects, an effect observed in rat offspring, occurred with the children of two out of seven female DuPont employees from the Washington Works facility from 1979 to 1981.[9][121] Bucky Bailey is one of the affected individuals, however, DuPont does not accept any liability from the toxicity of PFOA.[122] While 3M sent DuPont results from a study that showed birth defects to rats administered PFOA and DuPont moved the women out of the Teflon production unit,[9] subsequent animal testing led DuPont to conclude there was no reproductive risk to women, and they were returned to the production unit.[123] However, data released in March 2009 on the community around DuPont's Washington Works plant showed "a modest, imprecise indication of an elevation in risk...above the 90th percentile...based on 12 cases in the uppermost category" which was deemed "suggestive of a possible relationship" between PFOA exposure and birth defects.[124][125]
Legal actions
Industry and legal actions
DuPont has used PFOA for over 50 years at its Washington Works plant near Parkersburg, WV. Area residents sued DuPont in August 2001, and claimed DuPont released PFOA in excess of their community guideline of 1 part per billion resulting in lower property values and increased risk of illness.[9] The class was certified by Wood Circuit Court Judge George W. Hill.[126] As part of the settlement, DuPont is paying for blood tests and health surveys of residents believed to be affected. Participants number 69,030 in the study, which will be reviewed by three epidemiologists—the C8 Science Panel—to determine if any health effects are the likely result of exposure.
On December 13, 2005, DuPont announced a settlement with the EPA in which DuPont will pay US$10.25 million in fines and an additional US$6.25 million for two supplemental environmental projects without any admission of liability.[127]
On September 30, 2008, Chief Judge Joseph R. Goodwin of the United States District Court for the Southern District of West Virginia denied the certification of a class of Parkersburg residents exposed to PFOA from DuPont's facility because they did not "show the common individual injuries needed to certify a class action."[128] On September 28, 2009, Judge Goodwin dismissed the claims of those residents except for medical monitoring.[126][129]
U.S. federal government actions
In 2002, a panel of toxicologists, including several from the USEPA, proposed a level of 150 parts per billion for drinking water in the PFOA contaminated area around DuPont's Washington Works WV plant; this level was much higher than any known environmental concentration.[32]
In July 2004, the USEPA filed a suit against DuPont alleging "widespread contamination" of PFOA near the Parkersburg, WV, plant "at levels exceeding the company’s community exposure guidelines;" the suit also alleged that "DuPont had—over a 20 year period—repeatedly failed to submit information on adverse effects (in particular, information on liver enzyme alterations and birth defects in offspring of female Parkersburg workers)."[9]
In October 2005, a USFDA study was published revealing PFOA and PFOA precursor chemicals in food contact and PTFE products.[75]
On January 25, 2006, the USEPA announced a voluntary program with several chemical companies to reduce PFOA and PFOA precursor emissions by the year 2015.[130]
On February 15, 2005, the USEPA's Science Advisory Board (SAB) voted to recommended that PFOA should be considered a "likely human carcinogen."[131]
On May 26, 2006, the USEPA's SAB addressed a letter to Stephen L. Johnson. Three-quarters of advisers thought the stronger "likely to be carcinogenic" descriptor was warranted, in opposition to the USEPA's own PFOA hazard descriptor of "suggestive evidence of carcinogenicity, but not sufficient to assess human carcinogenic potential."[132]
On November 21, 2006, the USEPA ordered DuPont company to offer alternative drinking water or treatment for public or private water users living near DuPont's Washington Works plant in West Virginia (and in Ohio), if the level of PFOA detected in drinking water is equal to or greater than 0.5 parts per billion. This measure sharply lowered the previous action level of 150 parts per billion that was established in March 2002.[133]
According to a May 23, 2007, Environmental Science & Technology Online article, U.S. Food and Drug Administration research regarding food contact papers as a potential source of PFOA to humans is ongoing.[61]
In November 2007, the Centers for Disease Control and Prevention (CDC) published data on PFOA concentrations comparing 1999–2000 vs. 2003–2004 NHANES samples.[55]
On January 15, 2009, the USEPA set a provisional health advisory level of 0.4 parts per billion in drinking water.[82]
U.S. state government actions
On February 13, 2007, the New Jersey Department of Environmental Protection issued a preliminary health-based guidance level of 0.04 parts per billion in drinking water, due to PFOA being found at "elevated levels in the system's drinking water near DuPont's massive Chambers Works chemical plant."[134]
On March 1, 2007, the Minnesota Department of Health lowered its Health Based Value for PFOA in drinking water from 1.0 parts per billion to 0.5 parts per billion,[135] where "the sources are landfilled industrial wastes from a 3M manufacturing plant."[134]
European action
PFOA contaminated waste was incorporated into soil improver and spread on agricultural land in Germany, leading to PFOA drinking water contamination of up to 0.519 parts per billion.[136][137] The German Federal Environmental Agency issued guidelines for the sum of PFOA and PFOS concentrations in drinking water: 0.1 parts per billion for precaution and 0.3 parts per billion for a threshold.[97] Residents were found to have a 6–8 factor increase of PFOA serum levels over unexposed Germans, with average PFOA concentrations in the 22–27 parts per billion range.[46] An expert panel concluded that "concentrations were considered too low to cause overt adverse health effects in the exposed population."[97]
See also
References
- ^ a b c d Record of Perfluorooctanoic acid in the GESTIS Substance Database from the IFA, accessed on 5 November 2008
- ^ a b c d e f g Prevedouros K, Cousins IT, Buck RC, Korzeniowski SH (December 2006). "Sources, fate and transport of perfluorocarboxylates". Environ. Sci. Technol. 40 (1): 32–44. doi:10.1021/es0512475. PMID 16433330.
- ^ Goss KU (July 2008). "The pKa values of PFOA and other highly fluorinated carboxylic acids". Environ. Sci. Technol. 42 (2): 456–458. doi:10.1021/es702192c. PMID 18284146.
- ^ Cheng J, Psillakis E, Hoffmann MR, Colussi AJ (July 2009). "Acid dissociation versus molecular association of perfluoroalkyl oxoacids: Environmental implications". J. Phys. Chem. A 113 (29): 8152–8156. doi:10.1021/jp9051352. PMID 19569653.
- ^ Rayne S, Forest K (June 2010). "Theoretical studies on the pKa values of perfluoroalkyl carboxylic acids". J. Mol. Struct. (Theochem) 949 (1–3): 60–69. doi:10.1016/j.theochem.2010.03.003.
- ^ a b Shankar, Anoop; Jie Xiao and Alan Ducatman (2011 Oct 15). "Perfluoroalkyl Chemicals and Chronic Kidney Disease in US Adults". American Journal of Epidemiology 174 (8): 893–900. doi:10.1093/aje/kwr171. PMID 2187360. PMID 2187360. http://aje.oxfordjournals.org/content/174/8/893.abstract. Retrieved 2011-10-10.
- ^ a b c d Emmett EA, Shofer FS, Zhang H, Freeman D, Desai C, Shaw LM (August 2006). "Community exposure to perfluorooctanoate: relationships between serum concentrations and exposure sources". J. Occup. Environ. Med. 48 (8): 759–70. doi:10.1097/01.jom.0000232486.07658.74. PMC 3038253. PMID 16902368. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3038253.
- ^ Arneson, Gerald J. (November 1961). "Toxicity of Teflon Dispersing Agents" (PDF). DuPont, Polychemicals Department, Research & Development Division, Experimental Station. http://www.defendingscience.org/case_studies/upload/1961-memo.pdf. Retrieved 2008-09-21.
- ^ a b c d e f Clapp, Richard; Polly Hoppin, Jyotsna Jagai, Sara Donahue. "Case Studies in Science Policy: Perfluorooctanoic Acid". Project on Scientific Knowledge and Public Policy (SKAPP). http://www.defendingscience.org/case_studies/perfluorooctanoic-acid.cfm. Retrieved 2008-12-19.
- ^ a b c Kennedy GL, Butenhoff JL, Olsen GW, et al. (2004). "The toxicology of perfluorooctanoate". Crit. Rev. Toxicol. 34 (4): 351–84. doi:10.1080/10408440490464705. PMID 15328768.
- ^ Giesy JP, Kannan K (April 2002). "Perfluorochemical surfactants in the environment". Environ. Sci. Technol. 36 (7): 146A–152A. doi:10.1021/es022253t. PMID 11999053.
- ^ a b Lau C, Butenhoff JL, Rogers JM (July 2004). "The developmental toxicity of perfluoroalkyl acids and their derivatives". Toxicol. Appl. Pharmacol. 198 (2): 231–41. doi:10.1016/j.taap.2003.11.031. PMID 15236955.
- ^ Ullah, Aziz (October 2006). "The Fluorochemical Dilemma: What the PFOS/PFOA fuss is all about" (PDF). Cleaning & Restoration. https://www.restorationindustry.org/buyersguide/FlurochemicalsOct06.pdf. Retrieved 2008-09-24.
- ^ Lee, Jennifer 8. (15 April 2003). "E.P.A. Orders Companies to Examine Effects of Chemicals". The New York Times. http://www.nytimes.com/2003/04/15/science/epa-orders-companies-to-examine-effects-of-chemicals.html?pagewanted=2. Retrieved 15 May 2009.
- ^ "3M United States: PFOS PFOA: What is 3M Doing?". 3M Company. http://solutions.3m.com/wps/portal/3M/en_US/PFOS/PFOA/Information/Action. Retrieved 2009-01-05.
- ^ Weber, Joseph (5 June 2000). "3M's Big Cleanup – Why it decided to pull the plug on its best-selling stain repellent". Business Week (New York: McGraw-Hill) (3684): 96.
- ^ a b Ward, Jr., Ken (7 November 2008). "DuPont finds high C8 in Chinese workers". The Charleston Gazette. http://sundaygazettemail.com/News/200811060596?page=1&build=cache. Retrieved 6 January 2009.
- ^ a b c d e f g h Savu, P (1994). "Fluorinated Higher Carboxylic Acids". Kirk-Othmer Encyclopedia of Chemical Terminology. John Wiley & Sons, Inc. doi:10.1002/0471238961.0612211519012221.a01.
- ^ a b Goeden, Helen (June 2008). "Issues and Needs for PFAA Exposure and Health Research: A State Perspective" (PDF). PFAA Days II, Minnesota Department of Health. U.S. EPA - Research Triangle Park. http://www.health.state.mn.us/divs/eh/hazardous/topics/pfcs/pfaapresent.pdf. Retrieved 2008-12-02.
- ^ a b c Lehmler, HJ (March 2005). "Synthesis of environmentally relevant fluorinated surfactants—a review". Chemosphere 58 (11): 1471–96. doi:10.1016/j.chemosphere.2004.11.078. PMC 2587313. PMID 15694468. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2587313.
- ^ Ylinen M, Hanhijärvi H, Peura P, Rämö O (November 1985). "Quantitative gas chromatographic determination of perfluorooctanoic acid as the benzyl ester in plasma and urine". Arch. Environ. Contam. Toxicol. 14 (6): 713–7. doi:10.1007/BF01055778. PMID 4073944.
- ^ "PFOA in Norway TA-2354/2007". Norwegian Pollution Control Authority. 2007. p. 6. http://www.sft.no/publikasjoner/2354/ta2354.pdf. Retrieved 6 April 2009.
- ^ "Information on PFOA". DuPont. http://www2.dupont.com/PFOA/en_US/. Retrieved 23 May 2009.
- ^ Siegle, Lucy (11 October 2009). "Do environmentally friendly outdoor jackets exist?". The Observer (London). http://www.guardian.co.uk/environment/2009/oct/11/outdoor-clothing-ethical-living. Retrieved 25 October 2009.
- ^ a b Kudo N, Kawashima Y (May 2003). "Toxicity and toxicokinetics of perfluorooctanoic acid in humans and animals". J. Toxicol. Sci. 28 (2): 49–57. doi:10.2131/jts.28.49. PMID 12820537.
- ^ Kudo N, Suzuki-Nakajima E, Mitsumoto A, Kawashima Y (September 2006). "Responses of the liver to perfluorinated fatty acids with different carbon chain length in male and female mice:in relation to induction of hepatomegaly, peroxisomal beta-oxidation and microsomal 1-acylglycerophosphocholine acyltransferase". Biol. Pharm. Bull. 29 (9): 1952–7. doi:10.1248/bpb.29.1952. PMID 16946516.
- ^ Gordon SC (September 2010). "Toxicological evaluation of ammonium 4,8-dioxa-3H-perfluorononanoate, a new emulsifier to replace ammonium perfluorooctanoate in fluoropolymer manufacturing". Regul Toxicol Pharmacol 59 (1): 64–80. doi:10.1016/j.yrtph.2010.09.008. PMID 20875479.
- ^ Sandy, Martha. "Petition for Expedited CIC Consideration of Perfluorooctanic Acid (PFOA)". The State of California, Office of Environmental Health Hazard Assessment, Cancer Toxicology and Epidemiology Section, Reproductive and Cancer Hazard Assessment Branch. http://www.oehha.ca.gov/Prop65/public_meetings/pdf/PFOACIC%20Slides121206.pdf. Retrieved 2008-09-27.
- ^ a b c d Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J (October 2007). "Perfluoroalkyl acids: a review of monitoring and toxicological findings". Toxicol. Sci. 99 (2): 366–94. doi:10.1093/toxsci/kfm128. PMID 17519394. http://toxsci.oxfordjournals.org/cgi/reprint/99/2/366.pdf.
- ^ Michael McCoy (November 2008). "Dyneon Phasing Out Perfluorooctanoate". Chemical & Engineering News 86 (46): 26. doi:10.1021/cen-v086n033.p026. http://pubs.acs.org/isubscribe/journals/cen/86/i46/html/8646busc7.html.
- ^ "Learn More About DuPont Teflon". DuPont. http://www2.dupont.com/Teflon/en_US/keyword/pfoa.html?src=search_us_pfoa. Retrieved 16 May 2009.
- ^ a b c Renner, Rebecca (June 2003). "Concerns over common perfluorinated surfactant". Environ. Sci. Technol. 37 (11): 201A–2A. doi:10.1021/es032467q. PMID 12831000. http://pubs.acs.org/doi/abs/10.1021/es032467q.
- ^ G. Siegemund, W. Schwertfeger, A. Feiring, B. Smart, F. Behr, H. Vogel, B. McKusick (2005). "Fluorine Compounds, Organic". Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH.
- ^ USEPA (7 March 2006). "Premanufacture Notification Exemption for Polymers; Amendment of Polymer Exemption Rule to Exclude Certain Perfluorinated Polymers; Proposed Rule" (PDF). Federal Register 71 (44): 11490. http://edocket.access.gpo.gov/2006/pdf/06-2152.pdf.
- ^ a b Salager, Jean-Louis (2002). "FIRP Booklet # 300-A: Surfactants-Types and Uses". Universidad de los Andes Laboratory of Formulation, Interfaces Rheology, and Processes. pp. 44. http://nanoparticles.org/pdf/Salager-E300A.pdf. Retrieved 2008-09-07.
- ^ a b Guo Z, Liu X, Krebs KA (March 2009). "Perfluorocarboxylic Acid Content in 116 Articles of Commerce" (PDF). USEPA. p. 40. http://www.epa.gov/nrmrl/pubs/600r09033/600r09033.pdf.
- ^ a b Lemal DM (January 2004). "Perspective on fluorocarbon chemistry". J. Org. Chem. 69 (1): 1–11. doi:10.1021/jo0302556. PMID 14703372.
- ^ a b c d e f Renner R (December 2008). "EPA finds record PFOS, PFOA levels in Alabama grazing fields". Environ. Sci. Technol. 43 (5): 1245–6. doi:10.1021/es803520c. PMID 19350885.
- ^ Cheng J, Psillakis E, Hoffmann MR, Colussi AJ (2009). "Acid Dissociation versus Molecular Association of Perfluoroalkyl Oxoacids: Environmental Implications". J. Phys. Chem. A 113 (29): 8152–6. doi:10.1021/jp9051352. PMID 19569653.
- ^ Cheng X, Klaassen CD (November 2008). "Perfluorocarboxylic acids induce cytochrome P450 enzymes in mouse liver through activation of PPAR-alpha and CAR transcription factors". Toxicol. Sci. 106 (1): 29–36. doi:10.1093/toxsci/kfn147. PMC 2563145. PMID 18648086. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2563145.
- ^ a b Conder JM, Hoke RA, De Wolf W, Russell MH, Buck RC (February 2008). "Are PFCAs bioaccumulative? A critical review and comparison with regulatory criteria and persistent lipophilic compounds". Environ. Sci. Technol. 42 (4): 995–1003. doi:10.1021/es070895g. PMID 18351063.
- ^ Bartell SM, Calafat AM, Lyu C, Kato K, Ryan PB, Steenland K (February 2010). "Rate of decline in serum PFOA concentrations after granular activated carbon filtration at two public water systems in Ohio and West Virginia". Environ. Health Perspect. 118 (2): 222–8. doi:10.1289/ehp.0901252. PMC 2831921. PMID 20123620. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2831921.
- ^ a b Steenland K, Fletcher T, Savitz DA (2010). "Epidemiologic Evidence on the Health Effects of Perfluorooctanoic Acid (PFOA)". Environ. Health Perspect. 118 (8): 1100–8. doi:10.1289/ehp.0901827. PMC 2920088. PMID 20423814. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2920088.
- ^ Brede E, Wilhelm M, Göen T, Müller J, Rauchfuss K, Kraft M, Hölzer J (June 2010). "Two-year follow-up biomonitoring pilot study of residents' and controls' PFC plasma levels after PFOA reduction in public water system in Arnsberg, Germany". Int J Hyg Environ Health 213 (3): 217–23. doi:10.1016/j.ijheh.2010.03.007. PMID 20488749.
- ^ a b Melzer D, Rice N, Depledge MH, Henley WE, Galloway TS (2010). "Association Between Serum Perfluoroctanoic Acid (PFOA) and Thyroid Disease in the NHANES Study". Environ. Health Perspect. 118 (5): 686–92. doi:10.1289/ehp.0901584. PMC 2866686. PMID 20089479. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2866686.
- ^ a b c d e f Betts KS (May 2007). "Perfluoroalkyl acids: what is the evidence telling us?". Environ. Health Perspect. 115 (5): A250–6. doi:10.1289/ehp.115-a250. PMC 1867999. PMID 17520044. http://www.ehponline.org/members/2007/115-5/focus.html.
- ^ Yamashita N, Kannan K, Taniyasu S, Horii Y, Petrick G, Gamo T (2005). "A global survey of perfluorinated acids in oceans". Mar. Pollut. Bull. 51 (8–12): 658–68. doi:10.1016/j.marpolbul.2005.04.026. PMID 15913661.
- ^ Renner, Rebecca (June 2008). "Aerosols complicate PFOA picture". Environ. Sci. Technol. 42 (11): 3908. doi:10.1021/es087117o. PMID 18589941. http://pubs.acs.org/doi/abs/10.1021/es087117o.
- ^ a b Houde M, Martin JW, Letcher RJ, Solomon KR, Muir DC (June 2006). "Biological monitoring of polyfluoroalkyl substances: A review". Environ. Sci. Technol. 40 (11): 3463–73. doi:10.1021/es052580b. PMID 16786681.
- ^ Butt CM, Berger U, Bossi R, Tomy GT (May 2010). "Levels and trends of poly- and perfluorinated compounds in the arctic environment". Sci Total Environ 408 (15): 2936–65. doi:10.1016/j.scitotenv.2010.03.015. PMID 20493516.
- ^ Vestergren R, Cousins IT (August 2009). "Tracking the pathways of human exposure to perfluorocarboxylates". Environ. Sci. Technol. 43 (15): 5565–75. doi:10.1021/es900228k. PMID 19731646.
- ^ Calafat AM, Needham LL, Kuklenyik Z, Reidy JA, Tully JS, Aguilar-Villalobos M, Naeher LP (April 2006). "Perfluorinated chemicals in selected residents of the American continent". Chemosphere 63 (3): 490–6. doi:10.1016/j.chemosphere.2005.08.028. PMID 16213555.
- ^ Harada KH, Yang HR, Moon CS, Hung NN, Hitomi T, Inoue K, Niisoe T, Watanabe T, Kamiyama S, Takenaka K, Kim MY, Watanabe K, Takasuga T, Koizumi A (April 2010). "Levels of perfluorooctane sulfonate and perfluorooctanoic acid in female serum samples from Japan in 2008, Korea in 1994–2008 and Vietnam in 2007–2008". Chemosphere 79 (3): 314–9. doi:10.1016/j.chemosphere.2010.01.027. PMID 20149408.
- ^ Hemat H, Wilhelm M, Völkel W, Mosch C, Fromme H, Wittsiepe J (July 2010). "Low serum levels of perfluorooctanoic acid (PFOA), perfluorooctane sulfonate (PFOS) and perfluorohexane sulfonate (PFHxS) in children and adults from Afghanistan". Sci. Total Environ. 408 (16): 3493–5. doi:10.1016/j.scitotenv.2010.04.040. PMID 20471065.
- ^ a b c Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL (November 2007). "Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000". Environ. Health Perspect. 115 (11): 1596–602. doi:10.1289/ehp.10598. PMC 2072821. PMID 18007991. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2072821.
- ^ Renner, Rebecca (2008). "PFOS phaseout pays off". Environ. Sci. Technol. 42 (13): 4618. doi:10.1021/es0871614. PMID 18677976. http://pubs.acs.org/doi/abs/10.1021/es0871614.
- ^ Fuchs, Erin and Pam Sohn (10 February 2008). "Study finds high levels of stain-resistance ingredient in Conasauga River". Chattanooga Times Free Press. http://www.timesfreepress.com/news/2008/feb/10/epa-finds-high-levels-stain-resistance-ingredient. Retrieved 4 October 2008.
- ^ Clara M, Scheffknecht C, Scharf S, Weiss S, Gans O (2008). "Emissions of perfluorinated alkylated substances (PFAS) from point sources—identification of relevant branches". Water Sci. Technol. 58 (1): 59–66. doi:10.2166/wst.2008.641. PMID 18653937.
- ^ Lin AY, Panchangam SC, Lo CC (April 2009). "The impact of semiconductor, electronics and optoelectronic industries on downstream perfluorinated chemical contamination in Taiwanese rivers". Environ. Pollut. 157 (4): 1365–72. doi:10.1016/j.envpol.2008.11.033. PMID 19117653.
- ^ "Substance flow analysis for Switzerland: Perfluorinated surfactants perfluorooctanesulfonate (PFOS) and perfluorooctanoic acid (PFOA)". The Swiss Federal Office for the Environment (FOEN). 2009. http://www.environment-switzerland.ch/uw-0922-e. Retrieved 4 November 2010.
- ^ a b c d Renner, Rebecca (2007). "PFOA in people". Environ. Sci. Technol. 41 (13): 4497–500. doi:10.1021/es0725697. PMID 17695887.
- ^ D'eon JC, Hurley MD, Wallington TJ, Mabury SA (March 2006). "Atmospheric chemistry of N-methyl perfluorobutane sulfonamidoethanol, C4F9SO2N(CH3)CH2CH2OH: kinetics and mechanism of reaction with OH". Environ. Sci. Technol. 40 (6): 1862–8. doi:10.1021/es0520767. PMID 16570609.
- ^ Ellis DA, Mabury SA, Martin JW, Muir DC (July 2001). "Thermolysis of fluoropolymers as a potential source of halogenated organic acids in the environment". Nature 412 (6844): 321–4. doi:10.1038/35085548. PMID 11460160.
- ^ Ellis DA, Martin JW, Muir DC, Mabury SA (June 2003). "The use of 19F NMR and mass spectrometry for the elucidation of novel fluorinated acids and atmospheric fluoroacid precursors evolved in the thermolysis of fluoropolymers". Analyst 128 (6): 756–64. doi:10.1039/b212658c. PMID 12866900.
- ^ "Lists of PFOS, PFAS, PFOA, PFCA, related compounds and chemicals that may degrade to PFCA" (PDF). Environment Directorate-Joint Meeting of the Chemicals Committee and the Working Party on Chemicals, Pesticides, and Biotechnology, Organisation for Economic Co-operation and Development. 2007-08-21. http://www.olis.oecd.org/olis/2006doc.nsf/LinkTo/NT00000F9A/$FILE/JT03231059.PDF. Retrieved 2008-09-19.
- ^ Schultz MM, Higgins CP, Huset CA, Luthy RG, Barofsky DF, Field JA (December 2006). "Fluorochemical mass flows in a municipal wastewater treatment facility". Environ. Sci. Technol. 40 (23): 7350–7. doi:10.1021/es061025m. PMC 2556954. PMID 17180988. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2556954.
- ^ Renner, Rebecca (2008). "Do perfluoropolymers biodegrade into PFOA?". Environ. Sci. Technol. 42 (3): 648–50. doi:10.1021/es087093l. PMID 18323078.
- ^ Schecter A, Colacino J, Haffner D, Patel K, Opel M, Päpke O, Birnbaum L (2010). "Perfluorinated Compounds, Polychlorinated Biphenyl, and Organochlorine Pesticide Contamination in Composite Food Samples from Dallas, Texas". Environ. Health Perspect. 118 (6): 796–802. doi:10.1289/ehp.0901347. PMC 2898856. PMID 20146964. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2898856.
- ^ Langer V, Dreyer A, Ebinghaus R (November 2010). "Polyfluorinated compounds in residential and nonresidential indoor air". Environ. Sci. Technol. 44 (21): 8075–81. doi:10.1021/es102384z. PMID 20925396.
- ^ D'eon JC, Mabury SA (2010). "Exploring Indirect Sources of Human Exposure to Perfluoroalkyl Carboxylates (PFCAs): Evaluating Uptake, Elimination and Biotransformation of Polyfluoroalkyl Phosphate Esters (PAPs) in the Rat". Environ Health Perspect 119 (3): 344–350. doi:10.1289/ehp.1002409. PMC 3059997. PMID 21059488. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3059997.
- ^ Post, Gloria; Stern, Alan; Murphy, Eileen. "Guidance for PFOA in Drinking Water at Pennsgrove Water Supply Company" (PDF). State of New Jersey, Department of Environmental Protection, Division of Science, Research and Technology. p. 2. http://www.state.nj.us/dep/watersupply/pfoa_dwguidance.pdf. Retrieved 7 June 2009.
- ^ Johnson, Mark. "Evaluation of Methodologies for Deriving Health-Based Values for PFCs in Drinking Water" (PDF). Agency for Toxic Substances and Disease Registry. pp. 20, 37. http://www.health.state.mn.us/divs/eh/hazardous/topics/pfcworkshop0507/pfcs_cdcatsdr.pdf. Retrieved 7 June 2009.
- ^ "Information on PFOA". DuPont. http://www2.dupont.com/PFOA/en_US/. Retrieved 14 February 2009.
- ^ a b Renner, Rebecca (January 2006). "It’s in the microwave popcorn, not the Teflon pan". Environ. Sci. Technol. 40 (1): 4. doi:10.1021/es062599u.
- ^ a b c Begley TH, White K, Honigfort P, Twaroski ML, Neches R, Walker RA (October 2005). "Perfluorochemicals: potential sources of and migration from food packaging". Food Addit. Contam. 22 (10): 1023–31. doi:10.1080/02652030500183474. PMID 16227186.
- ^ Weise, Elizabeth (16 November 2005). "Engineer: DuPont hid facts about paper coating". USA Today. http://www.usatoday.com/money/companies/management/2005-11-16-dupont-usat_x.htm. Retrieved 19 September 2008.
- ^ "Teflon firm faces fresh lawsuit". BBC News. 19 July 2005. http://news.bbc.co.uk/2/hi/business/4697939.stm. Retrieved 24 January 2009.
- ^ "PFOA in Norway TA-2354/2007". Norwegian Pollution Control Authority. 2007. p. 18. http://www.sft.no/publikasjoner/2354/ta2354.pdf. Retrieved 29 August 2009.
- ^ Trudel D, Horowitz L, Wormuth M, Scheringer M, Cousins IT, Hungerbühler K (April 2008). "Estimating consumer exposure to PFOS and PFOA". Risk Anal. 28 (2): 251–69. doi:10.1111/j.1539-6924.2008.01017.x. PMID 18419647.
- ^ "Nonstick pans: Nonstick coating risks". Consumer Reports. http://www.consumerreports.org/cro/home-garden/kitchen/cookware-bakeware-cutlery/nonstick-pans-6-07/overview/0607_pans_ov_1.htm. Retrieved 4 July 2009.
- ^ a b c d Ward, Jr., Ken (17 January 2009). "EPA's C8 advisory does not address long-term risks". The Charleston Gazette. http://wvgazette.com/News/200901160363?page=1&build=cache. Retrieved 8 February 2009.
- ^ a b c Finn, Scott (15 January 2009). "Bush EPA sets so-called safe level of C8 in drinking water". West Virginia Public Broadcasting. http://www.wvpubcast.org/newsarticle.aspx?id=7516. Retrieved 18 January 2009.
- ^ "Perfluorochemical Contamination of Biosolids Near Decatur, Alabama". United States Environmental Protection Agency. http://www.epa.gov/region4/water/PFCindex.html. Retrieved 12 June 2010.
- ^ Eilperin, Juliet (17 January 2009). "Level Set for Chemical In Nonstick Products". The Washington Post. http://www.washingtonpost.com/wp-dyn/content/article/2009/01/16/AR2009011604415.html. Retrieved 8 February 2009.
- ^ a b "Chemical Used to Make Non-Stick Coatings Harmful to Health". Environment News Service. 13 May 2008. http://www.ens-newswire.com/ens/may2008/2008-05-13-093.asp. Retrieved 19 October 2008.
- ^ Cheryl Hogue (September 2008). "California Chemical Legislation: State's new laws on chemicals could presage federal action". Chemical & Engineering News 86 (36): 9. http://pubs.acs.org/cen/news/86/i36/8636notw5.html.
- ^ a b "Calif. law establishes chemical review". San Francisco Chronicle (Associated Press). 29 September 2008. http://www.sfgate.com/cgi-bin/article.cgi?f=/n/a/2008/09/29/state/n121857D71.DTL&type=health. Retrieved 15 February 2009.[dead link]
- ^ Stokstad E (January 2006). "Environmental research—DuPont settlement to fund test of potential toxics". Science 311 (5757): 26–7. doi:10.1126/science.311.5757.26a. PMID 16400117.
- ^ Betts K (November 2007). "PFOS and PFOA in humans: new study links prenatal exposure to lower birth weight". Environ. Health Perspect. 115 (11): A550. doi:10.1289/ehp.115-a550a. PMC 2072861. PMID 18007977. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2072861.
- ^ Benninghoff, Abby D. (29 January 2009). "PFOA slows breast development in mice exposed via mom". Environmental Health News. http://www.environmentalhealthnews.org/ehs/newscience/pfoa-impairs-breast-development-in-mice. Retrieved 8 February 2009.
- ^ Biegel LB, Hurtt ME, Frame SR, O'Connor JC, Cook JC (March 2001). "Mechanisms of extrahepatic tumor induction by peroxisome proliferators in male CD rats". Toxicol. Sci. 60 (1): 44–55. doi:10.1093/toxsci/60.1.44. PMID 11222872. http://toxsci.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=11222872.
- ^ a b Hood E (August 2008). "Alternative Mechanism for PFOA?: Trout Studies Shed Light on Liver Effects". Environ. Health Perspect. 116 (8): A351. doi:10.1289/ehp.116-a351b. PMC 2516576. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2516576.
- ^ Hines EP, White SS, Stanko JP, Gibbs-Flournoy EA, Lau C, Fenton SE (May 2009). "Phenotypic dichotomy following developmental exposure to perfluorooctanoic acid (PFOA) in female CD-1 mice: Low doses induce elevated serum leptin and insulin, and overweight in mid-life". Mol. Cell. Endocrinol. 304 (1–2): 97–105. doi:10.1016/j.mce.2009.02.021. PMID 19433254.
- ^ Takagi A, Sai K, Umemura T, Hasegawa R, Kurokawa Y (April 1991). "Short-term exposure to the peroxisome proliferators, perfluorooctanoic acid and perfluorodecanoic acid, causes significant increase of 8-hydroxydeoxyguanosine in liver DNA of rats". Cancer Lett. 57 (1): 55–60. doi:10.1016/0304-3835(91)90063-N. PMID 2025879. http://linkinghub.elsevier.com/retrieve/pii/0304-3835(91)90063-N.
- ^ Upham BL, Park JS, Babica P, Sovadinova I, Rummel AM, Trosko JE, Hirose A, Hasegawa R, Kanno J, Sai K (April 2009). "Structure-activity-dependent regulation of cell communication by perfluorinated fatty acids using in vivo and in vitro model systems". Environ. Health Perspect. 117 (4): 545–51. doi:10.1289/ehp.11728. PMC 2679597. PMID 19440492. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2679597.
- ^ "Assessment of PFOA in the drinking water of the German Hochsauerlandkreis" (PDF). Drinking Water Commission (Trinkwasserkommission) of the German Ministry of Health at the Federal Environment Agency. pp. 2–3. http://www.umweltbundesamt.de/uba-info-presse-e/hintergrund/pft-in-drinking-water.pdf. Retrieved 12 June 2009.
- ^ a b c Roos PH, Angerer J, Dieter H, Wilhelm M, Wölfle D, Hengstler JG (January 2008). "Perfluorinated compounds (PFC) hit the headlines: meeting report on a satellite symposium of the annual meeting of the German Society of Toxicology". Arch. Toxicol. 82 (1): 57–9. doi:10.1007/s00204-007-0225-2. PMID 17687546.
- ^ Olsen GW, Mair DC, Church TR, et al. (July 2008). "Decline in perfluorooctanesulfonate and other polyfluoroalkyl chemicals in American Red Cross adult blood donors, 2000–2006". Environ. Sci. Technol. 42 (13): 4989–95. doi:10.1021/es800071x. PMID 18678038.
- ^ Olsen GW, Burris JM, Burlew MM, Mandel JH (November 2000). "Plasma cholecystokinin and hepatic enzymes, cholesterol and lipoproteins in ammonium perfluorooctanoate production workers". Drug Chem Toxicol 23 (4): 603–20. doi:10.1081/DCT-100101973. PMID 11071397.
- ^ Fei C, McLaughlin JK, Lipworth L, Olsen J (January 2009). "Maternal levels of perfluorinated chemicals and subfecundity". Hum. Reprod. 1 (1): 1–6. doi:10.1093/humrep/den490. PMID 19176540. http://humrep.oxfordjournals.org/cgi/reprint/den490v1.
- ^ Joensen UN, Bossi R, Leffers H, Jensen AA, Skakkebaek NE, Jørgensen N (June 2009). "Do perfluoroalkyl compounds impair human semen quality?". Environ. Health Perspect. 117 (6): 923–7. doi:10.1289/ehp.0800517. PMC 2702407. PMID 19590684. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2702407.
- ^ Lin CY, Lin LY, Chiang CK, Wang WJ, Su YN, Hung KY, Chen PC (December 2009). "Investigation of the Associations Between Low-Dose Serum Perfluorinated Chemicals and Liver Enzymes in US Adults". Am. J. Gastroenterol. 105 (6): 1354–63. doi:10.1038/ajg.2009.707. PMID 20010922.
- ^ Nelson JW, Hatch EE, Webster, TF (2009). "Exposure to Polyfluoroalkyl Chemicals and Cholesterol, Body Weight, and Insulin Resistance in the General U.S. Population" (PDF). Environ. Health Perspect. 118 (2): 197–202. doi:10.1289/ehp.0901165. PMC 2831917. PMID 20123614. http://www.ehponline.org/members/2009/0901165/0901165.pdf.
- ^ Hoffman K, Webster TF, Weisskopf MG, Weinberg J, Vieira VM (2010). "Exposure to Polyfluoroalkyl Chemicals and Attention Deficit Hyperactivity Disorder in U.S. Children Aged 12–15 Years". Environ. Health Perspect. 118 (12): 1762–7. doi:10.1289/ehp.1001898. PMC 3002197. PMID 20551004. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3002197.
- ^ Ken Ward Jr.. "PFOA linked to ADHD and hormone disruption in kids". Blogs @ The Charleston Gazette. http://blogs.wvgazette.com/watchdog/2009/11/03/pfoa-linked-to-adhd-and-hormone-disruption-in-kids/. Retrieved 8 November 2009.
- ^ Pinney, Susan; Gayle C. Windham, Frank M. Biro, Larry H. Kushi, Lusine Yaghjyan, Antonia Calafat, Kayoko Kato, Paul Succop, M. Kathryn Brown, Ann Hernick, Robert Bornschein. "Perfluorooctanoic acid (PFOA) and Pubertal Maturation in Young Girls". http://isee.conference-services.net/reports/template/onetextabstract.xml?xsl=template/onetextabstract.xsl&conferenceID=1651&abstractID=312130. Retrieved 8 November 2009.
- ^ "Patterns of age of puberty among children in the Mid-Ohio Valley in relation to Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS)" (PDF). C8 Science Panel. http://www.c8sciencepanel.org/pdfs/Status_Report_C8_and_puberty_27Sept2010.pdf. Retrieved 21 October 2010.
- ^ Fei C, McLaughlin JK, Tarone RE, Olsen J (November 2007). "Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort". Environ. Health Perspect. 115 (11): 1677–82. doi:10.1289/ehp.10506. PMC 2072850. PMID 18008003. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2072850.
- ^ Apelberg BJ, Witter FR, Herbstman JB, Calafat AM, Halden RU, Needham LL, Goldman LR (November 2007). "Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth". Environ. Health Perspect. 115 (11): 1670–6. doi:10.1289/ehp.10334. PMC 2072847. PMID 18008002. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2072847.
- ^ Andersen CS, Fei C, Gamborg M, Nohr EA, Sørensen TI, Olsen J (October 2010). "Prenatal Exposures to Perfluorinated Chemicals and Anthropometric Measures in Infancy". Am J Epidemiol 172 (11): 1230–7. doi:10.1093/aje/kwq289. PMID 20940176.
- ^ Washino N, Saijo Y, Sasaki S, Kato S, Ban S, Konishi K, Ito R, Nakata A, Iwasaki Y, Saito K, Nakazawa H, Kishi R (April 2009). "Correlations between prenatal exposure to perfluorinated chemicals and reduced fetal growth". Environ. Health Perspect. 117 (4): 660–7. doi:10.1289/ehp.11681. PMC 2679613. PMID 19440508. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2679613.
- ^ Monroy R, Morrison K, Teo K, Atkinson S, Kubwabo C, Stewart B, Foster WG (September 2008). "Serum levels of perfluoroalkyl compounds in human maternal and umbilical cord blood samples". Environ, Res. 108 (1): 56–62. doi:10.1016/j.envres.2008.06.001. PMID 18649879.
- ^ Nolan LA, Nolan JM, Shofer FS, Rodway NV, Emmett EA (June 2009). "The relationship between birth weight, gestational age and perfluorooctanoic acid (PFOA)-contaminated public drinking water". Reprod. Toxicol. 27 (3–4): 231–8. doi:10.1016/j.reprotox.2008.11.001. PMC 3039136. PMID 19049861. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3039136.
- ^ Eriksen KT, Sørensen M, McLaughlin JK, Lipworth L, Tjønneland A, Overvad K, Raaschou-Nielsen O (April 2009). "Perfluorooctanoate and perfluorooctanesulfonate plasma levels and risk of cancer in the general Danish population". J. Natl. Cancer Inst. 101 (8): 605–9. doi:10.1093/jnci/djp041. PMID 19351918.
- ^ Fei C, McLaughlin JK, Lipworth L, Olsen J (November 2010). "Prenatal exposure to PFOA and PFOS and risk of hospitalization for infectious diseases in early childhood". Environ. Res. 110 (8): 773–7. doi:10.1016/j.envres.2010.08.004. PMID 20800832.
- ^ Fei C, Olsen J (2010). "Prenatal Exposure to Perfluorinated Chemicals and Behavioral or Coordination Problems at Age 7". Environ. Health Perspect. 119 (4): 573–578. doi:10.1289/ehp.1002026. PMC 3080943. PMID 21062688. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3080943.
- ^ Fei C, McLaughlin JK, Lipworth L, Olsen J (October 2008). "Prenatal exposure to perfluorooctanoate (PFOA) and perfluorooctanesulfonate (PFOS) and maternally reported developmental milestones in infancy". Environ. Health Perspect. 116 (10): 1391–5. doi:10.1289/ehp.11277. PMC 2569100. PMID 18941583. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2569100.
- ^ Steenland K, Tinker S, Frisbee S, Ducatman A, Vaccarino V (November 2009). "Association of perfluorooctanoic acid and perfluorooctane sulfonate with serum lipids among adults living near a chemical plant". Am. J. Epidemiol. 170 (10): 1268–78. doi:10.1093/aje/kwp279. PMID 19846564.
- ^ Frisbee SJ, Brooks AP, Maher A, Flensborg P, Arnold S, Fletcher T, Steenland K, Shankar A, Knox SS, Pollard C, Halverson JA, Vieira VM, Jin C, Leyden KM, Ducatman AM (December 2009). "The C8 health project: design, methods, and participants". Environ. Health Perspect. 117 (12): 1873–82. doi:10.1289/ehp.0800379. PMC 2799461. PMID 20049206. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2799461.
- ^ "Timeline". C8 Science Panel. http://www.c8sciencepanel.org/timeline.html. Retrieved 9 June 2011.
- ^ Cortese, Amy (8 August 2004). "DuPont, Now in the Frying Pan". The New York Times: p. 3. http://query.nytimes.com/gst/fullpage.html?res=9C05EFDA133CF93BA3575BC0A9629C8B63. Retrieved 30 December 2008.
- ^ Summers, Chris (7 October 2004). "Teflon's sticky situation". BBC News. http://news.bbc.co.uk/2/hi/uk_news/magazine/3697324.stm. Retrieved 30 December 2008.
- ^ "Biomonitoring—EPA Needs to Coordinate Its Research Strategy and Clarify Its Authority to Obtain Biomonitoring Data" (PDF). United States Government Accountability Office. April 2009. pp. 19–20. http://www.gao.gov/new.items/d09353.pdf. Retrieved 19 June 2009.
- ^ "Relationship of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) with pregnancy outcome among women with elevated community exposure to PFOA" (PDF). C8 Science Panel. http://www.c8sciencepanel.org/pdfs/Status_Report_C8_and_Pregnancy_Outcomes_March2009.pdf. Retrieved 27 June 2009.
- ^ "C8 Science Panel Website – C8 Study Results – Status Reports". C8 Science Panel. http://www.c8sciencepanel.org/study_results.html. Retrieved 27 June 2009.
- ^ a b Ken Ward, Jr. (28 September 2009). "Federal judge throws out most of C8 suit against DuPont". The Charleston Gazette. http://www.wvgazette.com/News/200909280659.
- ^ Michael Janofsky (15 December 2005). "DuPont to Pay $16.5 Million for Unreported Risks". The New York Times. http://www.nytimes.com/2005/12/15/politics/15enviro.html. Retrieved 23 November 2009.
- ^ Goodwin, C.J. "Rhodes, et al. v. E.I. Du Pont De Nemours and Company" United States District Court for the Southern District of West Virginia. Case Number, 6:06-cv-530 (30 September 2008). Retrieved 12 October 2008.
- ^ http://www.wvsd.uscourts.gov/district/opinions/pdf/606cv00530ORDsummj.pdf
- ^ "2010/15 PFOA Stewardship Program; PFOA and Fluorinated Telomers". U.S. Environmental Protection Agency. http://www.epa.gov/opptintr/pfoa/pubs/pfoastewardship.htm. Retrieved 19 September 2008.[dead link]
- ^ Renner R; Christen, Kris (2006). "Scientists hail PFOA reduction plan". Environ. Sci. Technol. 40 (7): 2083. doi:10.1021/es062654z.
- ^ "SAB Review of EPA’s Draft Risk Assessment of Potential Human Health Effects Associated with PFOA and Its Salts" (PDF). U.S. Environmental Protection Agency Science Advisory Board. 2006-05-30. pp. 2. http://yosemite.epa.gov/sab/sabproduct.nsf/A3C83648E77252828525717F004B9099/$File/sab_06_006.pdf. Retrieved 2008-09-21.
- ^ Mid-Atlantic Enforcement (10 May 2007). "Fact Sheet: EPA, DuPont Agree on Measures to Protect Drinking Water Near the DuPont Washington Works". United States Environmental Protection Agency. Archived from the original on 18 January 2008. http://web.archive.org/web/20080118114054/http://www.epa.gov/region03/enforcement/dupont_factsheet.html. Retrieved 11 May 2008.
- ^ a b Renner R; Renner, Rebecca; Cooney, Catherine M.; Pelley, Janet; Chatterjee, Rhitu; Lubick, Naomi; Engelhaupt, Erika (May 2007). "New Jersey dives into PFOA water guidance". Environ. Sci. Technol. 41 (10): 3395–6. doi:10.1021/es072532m. PMID 17547148.
- ^ Minnesota Department of Health "Health officials issue new health guidelines for PFOA, PFOS" News Release (March 1, 2007).
- ^ Anke Schaefer; Barbara Booth; Naomi Lubick; Kellyn S. Betts (2006-12-01). "Perfluorinated surfactants contaminate German waters – Mislabeled waste in fertilizer leads to a water scandal". Environ. Sci. Technol. 40 (23): 7108–14. doi:10.1021/es062811u.
- ^ Skutlarek D, Exner M, Färber H (September 2006). "Perfluorinated surfactants in surface and drinking waters". Environ. Sci. Pollut. Res. Int. 13 (5): 299–307. doi:10.1065/espr2006.07.326. PMID 17067024. http://www.scientificjournals.com/sj/espr/Abstract/ArtikelId/8685.
External links
- USEPA: PFOA and Fluorotelomer page
- USEPA: Links to related progams and studies
- Sustained Outrage Blog – C8 (PFOA) Category published by the Charleston Gazette
- Callie Lyons blog on C8, author of Stain-Resistant, Nonstick, Waterproof, and Lethal: The Hidden Dangers of C8
- Perfluorooctanoic Acid (PFOA); Fluorinated Telomers enforceable consent agreement development
- Perfluorinated substances and their uses in Sweden
- Chain of Contamination: The Food Link, Perfluorinated Chemicals (PFCs) Incl. PFOS & PFOA
Categories:- Carboxylic acids
- Fatty acids
- Perfluorinated compounds
- Pollutants
- Anionic surfactants
Wikimedia Foundation. 2010.