- Tropone
-
Tropone[1] 
 Cyclohepta-2,4,6-trien-1-one
Cyclohepta-2,4,6-trien-1-oneIdentifiers CAS number 539-80-0 PubChem 10881 ChemSpider 10419 
Jmol-3D images Image 1 - O=C\1/C=C\C=C/C=C/1
Properties Molecular formula C7H6O Molar mass 106.12 g/mol Density 1.094 g/mL Boiling point 113 °C (15 mmHg)
Hazards Flash point >113 °C  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Tropone or 2,4,6-cycloheptatrien-1-one is an organic compound with some importance in organic chemistry as a non-benzenoid aromatic.[2] The compound consists of a ring of seven carbon atoms with three conjugated alkene groups and a ketone group. The related compound tropolone (2-hydroxy-2,4,6-cycloheptatrien-1-one) has an additional alcohol (or an enol including the double bond) group next to the ketone.
The tropone moiety can be found in biomolecules such as colchicine, stipitatic acid and hinokitiol.
Tropone has been known since 1951 and is also called cycloheptatrienylium oxide. The name tropolone was coined by M. J. S. Dewar in 1945 in connection to perceived aromatic properties.[3]
Contents
Properties
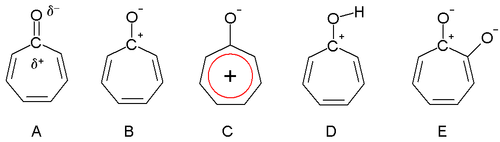
Dewar in 1945 proposed that tropones could have aromatic properties. The carbonyl group is polarized with a partial positive charge on the carbon atom (A) and a partial negative charge on oxygen. In an extreme case the carbon atom has a full positive charge (B) forming a tropylium ion ring which is an aromatic 6 electron system (C).
Tropolone is acidic (E) with a pKa of 7 which is in between that of phenol (10) and benzoic acid (4). The increased acidity compared to phenol is due to regular resonance stabilization. Tropones and to a lesser extent tropolones are also basic (D) and this is very much due to aromatic stabilization. This property can be observed in the ease of salt formation with acids. The dipole moment for tropone is 4.17 D compared to a value of only 3.04 D for cycloheptanone, which can also be taken as evidence for aromaticity.
Synthesis
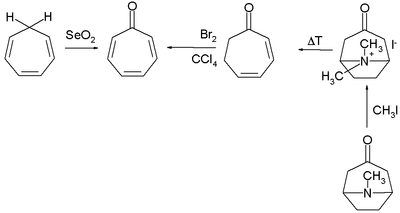
Numerous methods exist for the organic synthesis of tropones and its derivatives. Two selected methods for the synthesis of tropone are by selenium oxide oxidation of cycloheptatriene[4] and indirectly from tropinone by a Hofmann elimination and a bromination.
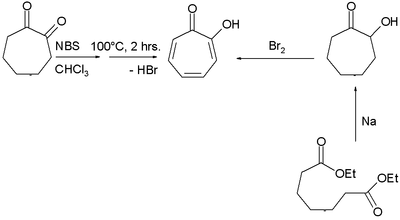
Two methods for the synthesis of tropolone are by bromination of 1,2-cycloheptanedione with N-bromosuccinimide followed by dehydrohalogenation at elevated temperatures and by acyloin condensation of the ethyl ester of pimelic acid the acyloin again followed by oxidation by bromine.
Reactions
- Tropone isomerizes to benzoic acid with potassium hydroxide at elevated temperature. Many derivatives also isomerize to the corresponding arene compounds.
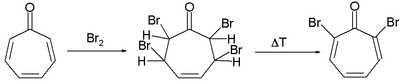
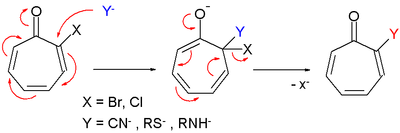
- Tropone reacts in electrophilic substitution for instance with bromine but the reaction proceeds through the 1,2-addition product and is not an electrophilic aromatic substitution.
- Tropone derivatives also react in nucleophilic substitution very much like in nucleophilic aromatic substitution.
- Tropone is a diene in a Diels-Alder reaction for instance with maleic anhydride
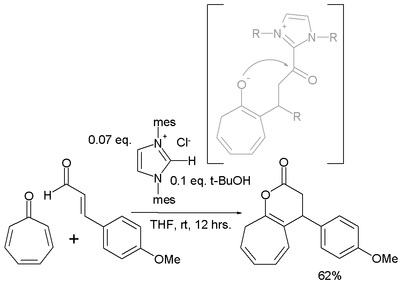
- Tropone is also found to react in an [8+3]annulation with a cinnamic aldehyde[5]
References
- ^ Tropone at Sigma-Aldrich
- ^ Tropones and Tropolones Peter L. Pauson Chem. Rev.; 1955; 55(1) pp 9 - 136; doi:10.1021/cr50001a002
- ^ M. J. S. Dewar (1945). "Structure of Stipitatic Acid". Nature 155 (3924): 50–51. doi:10.1038/155050b0.
- ^ organic Syntheses Coll. Vol. 9, p.396 (1998); Vol. 71, p.181 (1993) Online article
- ^ An N-Heterocyclic Carbene-Catalyzed [8 + 3] Annulation of Tropone and Enals via Homoenolate Vijay Nair, Manojkumar Poonoth, Sreekumar Vellalath, Eringathodi Suresh, and Rajasekaran Thirumalai J. Org. Chem.; 2006; 71(23) pp 8964 - 8965; (Note) doi:10.1021/jo0615706
Pharmacology: enzyme inhibition Class Substrate Oxidoreductase (EC 1)1.1 Aldose reductase · HMG-CoA reductase
1.13 Lipoxygenase
1.17 Xanthine oxidase · Ribonucleotide reductaseTransferase (EC 2)2.1 COMT · Thymidylate synthase
2.4 PARP
2.5 Dihydropteroate synthetase · Farnesyltransferase
2.6 GABA transaminase
2.7 Nucleotidyltransferase (Integrase, Reverse transcriptase) · Protein kinase (Tyrosine-kinase (Janus kinase))Hydrolase (EC 3)3.1 Phosphodiesterase · Acetylcholinesterase · Ribonuclease
3.2 Polygalacturonase · Neuraminidase · Alpha-glucosidase
3.4 Protease: Exopeptidase (Dipeptidyl peptidase-4, ACE) · Endopeptidase (Trypsin, Renin, Matrix metalloproteinase)
3.5 Histone deacetylase · Beta-lactamaseLyase (EC 4)4.1 Dopa decarboxylase
4.2 Carbonic anhydraseDopaminergics Reuptake inhibitors PlasmalemmalDAT inhibitorsPiperazines: DBL-583 • GBR-12,935 • Nefazodone • Vanoxerine; Piperidines: BTCP • Desoxypipradrol • Dextromethylphenidate • Difemetorex • Ethylphenidate • Methylnaphthidate • Methylphenidate • Phencyclidine • Pipradrol; Pyrrolidines: Diphenylprolinol • Methylenedioxypyrovalerone (MDPV) • Naphyrone • Prolintane • Pyrovalerone; Tropanes: β-CPPIT • Altropane • Brasofensine • CFT • Cocaine • Dichloropane • Difluoropine • FE-β-CPPIT • FP-β-CPPIT • Ioflupane (123I) • Iometopane • RTI-112 • RTI-113 • RTI-121 • RTI-126 • RTI-150 • RTI-177 • RTI-229 • RTI-336 • Tenocyclidine • Tesofensine • Troparil • Tropoxane • WF-11 • WF-23 • WF-31 • WF-33; Others: Adrafinil • Armodafinil • Amfonelic acid • Amineptine • Benzatropine (Benztropine) • Bromantane • BTQ • BTS-74,398 • Bupropion (Amfebutamone) • Ciclazindol • Diclofensine • Dimethocaine • Diphenylpyraline • Dizocilpine • DOV-102,677 • DOV-21,947 • DOV-216,303 • Etybenzatropine (Ethylbenztropine) • EXP-561 • Fencamine • Fencamfamine • Fezolamine • GYKI-52,895 • Indatraline • Ketamine • Lefetamine • Levophacetoperane • LR-5182 • Manifaxine • Mazindol • Medifoxamine • Mesocarb • Modafinil • Nefopam • Nomifensine • NS-2359 • O-2172 • Pridefrine • Propylamphetamine • Radafaxine • SEP-225,289 • SEP-227,162 • Sertraline • Sibutramine • Tametraline • Tedatioxetine • TripelennamineVMAT inhibitorsReleasing agents Morpholines: Fenbutrazate • Morazone • Phendimetrazine • Phenmetrazine; Oxazolines: 4-Methylaminorex (4-MAR, 4-MAX) • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone; Phenethylamines (also amphetamines, cathinones, phentermines, etc): 2-Hydroxyphenethylamine (2-OH-PEA) • 4-CAB • 4-Methylamphetamine (4-MA) • 4-Methylmethamphetamine (4-MMA) • Alfetamine • Amfecloral • Amfepentorex • Amfepramone • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • β-Methylphenethylamine (β-Me-PEA) • Benzodioxolylbutanamine (BDB) • Benzodioxolylhydroxybutanamine (BOH) • Benzphetamine • Buphedrone • Butylone • Cathine • Cathinone • Clobenzorex • Clortermine • D-Deprenyl • Dimethoxyamphetamine (DMA) • Dimethoxymethamphetamine (DMMA) • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, metamfepramone) • Ethcathinone (Ethylpropion) • Ethylamphetamine • Ethylbenzodioxolylbutanamine (EBDB) • Ethylone • Famprofazone • Fenethylline • Fenproporex • Flephedrone • Fludorex • Furfenorex • Hordenine • Lophophine (Homomyristicylamine) • Mefenorex • Mephedrone • Methamphetamine (Desoxyephedrine, Methedrine; Dextromethamphetamine, Levomethamphetamine) • Methcathinone (Methylpropion) • Methedrone • Methoxymethylenedioxyamphetamine (MMDA) • Methoxymethylenedioxymethamphetamine (MMDMA) • Methylbenzodioxolylbutanamine (MBDB) • Methylenedioxyamphetamine (MDA, tenamfetamine) • Methylenedioxyethylamphetamine (MDEA) • Methylenedioxyhydroxyamphetamine (MDOH) • Methylenedioxymethamphetamine (MDMA) • Methylenedioxymethylphenethylamine (MDMPEA, homarylamine) • Methylenedioxyphenethylamine (MDPEA, homopiperonylamine) • Methylone • Ortetamine • Parabromoamphetamine (PBA) • Parachloroamphetamine (PCA) • Parafluoroamphetamine (PFA) • Parafluoromethamphetamine (PFMA) • Parahydroxyamphetamine (PHA) • Paraiodoamphetamine (PIA) • Paredrine (Norpholedrine, Oxamphetamine) • Phenethylamine (PEA) • Pholedrine • Phenpromethamine • Prenylamine • Propylamphetamine • Tiflorex (Flutiorex) • Tyramine (TRA) • Xylopropamine • Zylofuramine; Piperazines: 2,5-Dimethoxy-4-bromobenzylpiperazine (2C-B-BZP) • Benzylpiperazine (BZP) • Methoxyphenylpiperazine (MeOPP, paraperazine) • Methylbenzylpiperazine (MBZP) • Methylenedioxybenzylpiperazine (MDBZP, piperonylpiperazine); Others: 2-Amino-1,2-dihydronaphthalene (2-ADN) • 2-Aminoindane (2-AI) • 2-Aminotetralin (2-AT) • 4-Benzylpiperidine (4-BP) • 5-IAI • Clofenciclan • Cyclopentamine • Cypenamine • Cyprodenate • Feprosidnine • Gilutensin • Heptaminol • Hexacyclonate • Indanylaminopropane (IAP) • Indanorex • Isometheptene • Methylhexanamine • Naphthylaminopropane (NAP) • Octodrine • Phthalimidopropiophenone • Propylhexedrine (Levopropylhexedrine) • Tuaminoheptane (Tuamine)Enzyme inhibitors PAH inhibitors3,4-DihydroxystyreneTH inhibitorsNonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima; MAO-B selective: D-Deprenyl • L-Deprenyl (Selegiline) • Ladostigil • Lazabemide • Milacemide • Pargyline • Rasagiline • SafinamideDBH inhibitorsOthers L-Phenylalanine → L-Tyrosine → L-DOPA (Levodopa)Ferrous iron (Fe2+) • Tetrahydrobiopterin • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal phosphate) • Vitamin B9 (Folic acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersActivity enhancers: Benzofuranylpropylaminopentane (BPAP) • Phenylpropylaminopentane (PPAP); Toxins: Oxidopamine (6-Hydroxydopamine)List of dopaminergic drugsCategories:- Alkenes
- Tropones
Wikimedia Foundation. 2010.