- Ergoline
-
Ergoline 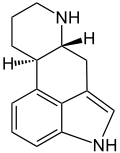
Systematic (IUPAC) name (6aR)- 4,6,6a,7,8,9,10,10a- octahydroindolo [4,3-fg] quinoline Clinical data Pregnancy cat. ? Legal status ? Identifiers CAS number 478-88-6 
ATC code ? PubChem CID 6857537 ChemSpider 5256873 
ChEBI CHEBI:38484 
Chemical data Formula C14H16N2 Mol. mass 212.29g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Ergoline is a chemical compound whose structural skeleton is contained in a diverse range of alkaloids including a few psychedelic drugs (e.g. lysergic acid and LSD). Ergoline derivatives are used clinically for the purpose of vasoconstriction (5-HT1 receptor agonists—ergotamine) and in the treatment of migraines (used with caffeine) and Parkinson's disease. Some ergoline alkaloids found in ergot fungi are implicated in the condition ergotism, which causes convulsive and gangrenous symptoms.
Contents
Uses
In addition to the naturally occurring ergonovine (used as an oxytocic) and ergotamine (a vasoconstrictor used to control migraine), synthetic derivatives of importance are the oxytocic methergine, the anti-migraine drugs dihydroergotamine and methysergide, hydergine (a mixture of dihydroergotoxine mesylates, INN: ergoline mesylates), and bromocriptine, used for numerous purposes including treatment of Parkinson's disease. Newer synthetic ergolines used for Parkinson's disease include pergolide and lisuride.
Perhaps the most famous ergoline derivative is the psychedelic drug LSD. Ergometrine and ergotamine are included as table I precursors in the United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances.[1]
Ergolines can pass into breast milk and should not be used during breastfeeding.[2] They are uterine contractors that can increase the risk of miscarriage during pregnancy.[3]
Natural occurrence
Ergoline alkaloids are found in lower fungi[3] and two species of flowering plants: the Mexican species Rivea corymbosa and Ipomoea violacea of the Convolvulaceae (morning glory) family, the seeds of which were identified as the psychedelic plant drugs known as "ololiuhqui" and "tlitliltzin"[verification needed][citation needed]. The principal alkaloids in the seeds are ergine and its optical isomer isoergine, with several other lysergic acid derivatives and clavines present in lesser amounts. The Hawaiian species Argyreia nervosa includes similar alkaloids. It is possible, though not proven, that ergine or isoergine are responsible for the hallucinogenic effects. There may be a fungal origin of the ergoline alkaloids also in the Convolvulaceae. Like the ergot alkaloids in some monocot plants, the ergoline alkaloids found in the plant Ipomoea asarifolia (Convolvulaceae) are produced by a seed-transmitted epiphytic clavicipitaceous fungus.[4]
Ergopeptines
Peptide ergot alkaloids are ergoline derivatives that contain a tripeptide moiety, comprising proline and α-hydroxy-α-amino acids, linked in a cyclol formation with the carboxyl carbon of proline.[5]
History
Ergoline alkaloids were first isolated from ergot, a fungus that infects grain and causes the disease ergotism. Ergot also has a long history of medicinal use, which led to attempts to characterize its activity chemically. This began in 1907 with the isolation by G. Barger and F. H. Carrin of ergotoxine, so-named since it appeared to exhibit more of the toxicity of ergot than its therapeutic qualities. With the isolation of ergotamine in 1918 by A. Stoll came the first therapeutic use of isolated ergoline alkaloids.
With the determination of the basic chemical structure of the ergot alkaloids in the early 1930s, an era of intensive exploration of synthetic derivatives began.
Ergoline derivatives
There are 3 main classes of ergoline derivatives, the water-soluble amides of lysergic acid, the water-insoluble ergopeptines (i.e., ergopeptides), and the clavine group.[3]
- Lysergic acid amides
Main article: Lysergamides- Ergine (LSA, D-lysergic acid amide, LAA, LA-111)
- IUPAC name: 9,10-didehydro-6-methylergoline-8beta-carboxamide
- CAS number: 478-94-4
- Ergonovine (ergobasine)
- INN: ergometrine
- IUPAC name: (8beta(S))−9,10-didehydro-N-(2-hydroxy-1-methylethyl)−6-methyl-ergoline-8-carboxamide
- CAS number: 60-79-7
- Methergine (ME-277)
- INN: methylergometrine
- IUPAC name: (8beta(S))−9,10-didehydro-N-(1-(hydroxymethyl)propyl)−6-methyl-ergoline-8-carboxamide
- CAS number: 113-42-8
- Methysergide (UML-491)
- INN: methysergide
- IUPAC name: (8beta)−9,10-didehydro-N-(1-(hydroxymethyl)propyl)−1,6-dimethyl-ergoline-8-carboxamide
- CAS number: 361-37-5
- LSD (D-lysergic acid diethylamide, LSD-25)
- INN: lysergide
- IUPAC name: (8beta)−9,10-didehydro-N,N-diethyl-6-methyl-ergoline-8-carboxamide
- CAS number: 50-37-3
- LSH (D-lysergic acid α-hydroxyethylamide)
- IUPAC name: 9,10-didehydro-N-(1-hydroxyethyl)-6-methylergoline-8-carboxamide
- CAS number: 3343-15-5
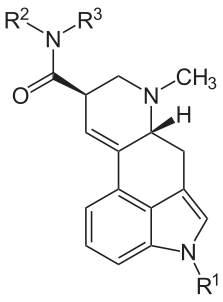
The relationship between these compounds is summarized in the following structural formula and table of substitutions.
Name R1 R2 R3 Ergine H H H Ergonovine H CH(CH3)CH2OH H Methergine H CH(CH2CH3)CH2OH H Methysergide CH3 CH(CH2CH3)CH2OH H LSD H CH2CH3 CH2CH3 - Peptide alkaloids
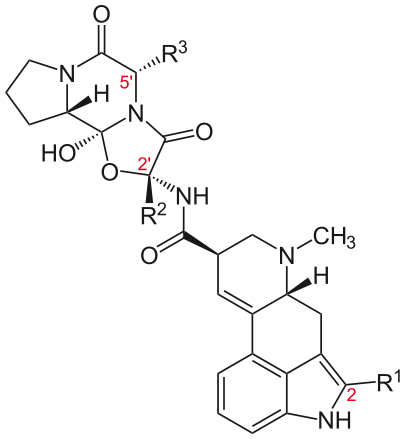
These compounds have a tripeptide structure attached to the basic ergoline ring, in the same location as the amide group of the lysergic acid derivatives. This tripeptide moiety contains an unusual cyclol bond >N-C(OH)< at the juncture between the two lactam rings. Some of the important ergopeptines (also known as ergopeptides) are summarized below. In addition to the following ergopeptines, a commonly encountered term is ergotoxine, which refers to a mixture of equal proportions of ergocristine, ergocornine and ergocryptine.
- Ergotamine
- IUPAC name: Ergotaman-3',6',18-trione, 12'-hydroxy-2'-methyl-5'-(phenylmethyl)-, (5'-alpha)- (9CI)
- CAS number: 113-15-5
- Ergocristine
- IUPAC name: Ergotaman-3',6',18-trione, 12'-hydroxy-2'-(1-methylethyl)−5'-(phenylmethyl)-, (5'-alpha)-
- CAS number: 511-08-0
- Ergocornine
- IUPAC name: Ergotaman-3',6',18-trione, 12'-hydroxy-2',5'-bis(1-methylethyl)-, (5'-alpha)-
- CAS number: 564-36-3
- Ergocryptine
- IUPAC name:Ergotaman-3',6',18-trione, 12'-hydroxy-2'-(1-methylethyl)−5'-(2-methylpropyl)-, (5'alpha)- (9CI)
- CAS number: 511-09-1
- Ergovaline
- IUPAC name: Ergotaman-3',6',18-trione, 12'-hydroxy-2'-methyl-5'-(1-methylethyl)-, (5'alpha)-
- CAS number: 2873-38-3
Name R1 R2 R3 Ergotamine CH3 benzyl Ergocristine CH(CH3)2 benzyl Ergocornine CH(CH3)2 CH(CH3)2 Ergocryptine CH(CH3)2 CH2CH(CH3)2 Bromocriptine Br CH(CH3)2 CH2CH(CH3)2 Ergovaline CH3 CH(CH3)2 - Clavines
A variety of modifications to the basic ergoline are seen in nature, for example agroclavine, elymoclavine, lysergol. Those deriving from dimethylergoline are referred to as clavines.
- Others
Some synthetic ergoline derivatives do not fall easily into any of the above groups. Some examples are:
- Pergolide (INN)
- IUPAC name: (8beta)−8-((methylthio)methyl)−6-propyl-ergoline
- CAS number: 66104-22-1
- Lisuride (INN)
- IUPAC name: 3-(9,10-didehydro-6-methylergolin-8alpha-yl)−1,1-diethylurea
- CAS number: 18016-80-3
See also
References
- ^ http://www.incb.org/pdf/e/list/red.pdf.
- ^ kidsgrowth.org --> Drugs and Other Substances in Breast Milk Retrieved on June 19, 2009.
- ^ a b c Schardl CL, Panaccione DG, Tudzynski P (2006). "Ergot alkaloids – biology and molecular biology". The Alkaloids: Chemistry and Biology. The Alkaloids: Chemistry and Biology 63: 45–86. doi:10.1016/S1099-4831(06)63002-2. ISBN 9780124695634. PMID 17133714.
- ^ Steiner, U; Ahimsa-Müller, MA; Markert, A; Kucht, S; Gross, J; Kauf, N; Kuzma, M; Zych, M et al. (2006). "Molecular characterization of a seed transmitted clavicipitaceous fungus occurring on dicotyledoneous plants (Convolvulaceae)". Planta 224 (3): 533–44. doi:10.1007/s00425-006-0241-0. PMID 16525783.
- ^ G. Floss, Heinz (1976). "Biosynthesis of Ergot Alkaloids and Related Compounds". Tetrahedron Report 32 (14): 873 to 912. doi:10.1016/0040-4020(76)85047-8.
External links
- The Ergot Alkaloids (A. T. Sneden)
- The Ergot Alkaloids Story (Z. Madlom)
- The Psychoactive Ergot Alkaloids and their occurrence in the Microfungi — M. P. Bock and D. G. Parbery
- Hofmann, A. Teonanácatl and Ololiuqui, two ancient magic drugs of Mexico Bulletin on Narcotics 1971 1 3
- TiHKAL (A & A Shulgin) #26
Ergolines Lysergic acid derivatives 2-Bromo-LSD (BOL-148) • Bromocriptine • Cabergoline • Dihydroergocornine • Dihydroergocristine • Dihydroergocryptine • Dihydroergometrine (Dihydroergonovine, Dihydroergobasine) • Dihydroergotamine • Dihydroergotoxine • Ergine (LSA; LA-111; Lysergamide) • Ergocornine • Ergocristine • Ergocryptine • Ergoloid • Ergometrine (Ergonovine, Ergobasine) • Ergometrinine • Ergotamine • Ergotoxine • Ergovaline • Lisuride • LSD • LSH • Lysergic Acid • Lysergic acid cyclobutylamide • Lysergic acid cyclopentylamide • Lysergic Acid Methyl Ester • Lysergol • Mesulergine • Metergoline • Methergine (Methylergometrine, Methylergonovine, Methylergobasine) • Methysergide • Pergolide • SyntometrinePsychedelic lysergamides AL-LAD • ALD-52 • BU-LAD • CYP-LAD • DAL • DAM-57 • Ergonovine • ETH-LAD • IP-LAD • LAE-32 • LSD • LPD-824 • LSM-775 • LSH • LSD-Pip • Lysergic Acid 2-Butylamide • Lysergic Acid 2,4-Dimethylazetidide • Lysergic Acid 3-Pentylamide • Methylergonovine • Methylisopropyllysergamide • MLD-41 • PARGY-LAD • PRO-LADOther ergolines ErgolineNatural sources Achnatherum robustum (Sleepy Grass) • Argyreia nervosa (Hawaiian Baby Woodrose) • Claviceps spp. (Ergot) • Ipomoea spp. (Morning Glory, Tlitliltzin, Badoh Negro) • Rivea corymbosa (Coaxihuitl, Ololiúqui)Categories:- Alkaloids
- Ergolines
Wikimedia Foundation. 2010.