- Cystic fibrosis
-
Cystic fibrosis Classification and external resources
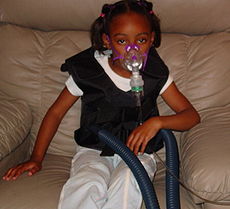
A breathing treatment for cystic fibrosis, using a mask nebuliser and a ThAIRapy VestICD-10 E84 ICD-9 277.0 OMIM 219700 DiseasesDB 3347 MedlinePlus 000107 eMedicine ped/535 MeSH D003550 Cystic fibrosis (also known as CF or mucoviscidosis) is a recessive multi-system genetic disease characterized by abnormal transport of chloride and sodium across epithelium, leading to thick, viscous secretions in the lungs, pancreas, liver, and intestine.[1]
The name cystic fibrosis refers to the characteristic scarring (fibrosis) and cyst formation within the pancreas, first recognized in the 1930s.[2] Difficulty breathing is the most serious symptom and results from frequent lung infections that are treated with, though not cured by, antibiotics and other medications. A multitude of other symptoms, including sinus infections, poor growth, diarrhea, and infertility result from the effects of CF on other parts of the body.
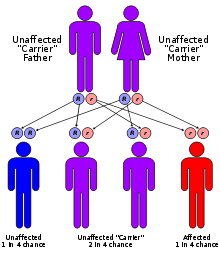
CF is caused by a mutation in the gene for the protein cystic fibrosis transmembrane conductance regulator (CFTR). This gene is required to regulate the components of sweat, digestive juices, and mucus. Although most people without CF have two working copies of the CFTR gene, only one is needed to prevent cystic fibrosis. CF develops when neither gene works normally and therefore has autosomal recessive inheritance.
CF is most common among Caucasians; one in 25 people of European descent carries one allele for CF.[3][4]
Ireland has both the highest incidence of CF in the world; 2.98 per 10,000 - and the highest carrier rate in the world with 1 in 19 individuals classed as carriers. Cystic fibrosis is Ireland's most common life-threatening inherited disease. Ireland also has the largest proportion of families with more than one child suffering from CF.[5][6][7]
Individuals with cystic fibrosis can be diagnosed before birth by genetic testing, or by a sweat test in early childhood. Ultimately, lung transplantation is often necessary as CF worsens.
Contents
Signs and symptoms
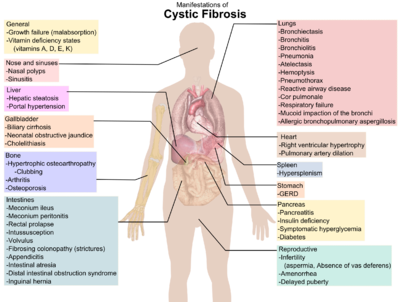 A diagram showing clinical manifestations of cystic fibrosis[8]
A diagram showing clinical manifestations of cystic fibrosis[8]
The hallmark symptoms of cystic fibrosis are salty tasting skin,[9] poor growth and poor weight gain despite a normal food intake,[10] accumulation of thick, sticky mucus,[11] frequent chest infections and coughing or shortness of breath.[12] Males can be infertile due to congenital absence of the vas deferens.[13] Symptoms often appear in infancy and childhood, such as bowel obstruction due to meconium ileus in newborn babies.[14] As the child grows, he or she will need to exercise to release mucus in the alveoli.[15] Ciliated epithelial cells in the patient have a mutated protein that leads to abnormally viscous mucus production.[11] The poor growth in children typically presents as an inability to gain weight or height at the same rate as their peers and is occasionally not diagnosed until investigation is initiated for poor growth. The causes of growth failure are multi-factorial and include chronic lung infection, poor absorption of nutrients through the gastrointestinal tract, and increased metabolic demand due to chronic illness.[10]
In rare cases, cystic fibrosis can manifest itself as a coagulation disorder. A double recessive allele is needed for cystic fibrosis to be apparent. Young children are especially sensitive to vitamin K malabsorptive disorders because only a very small amount of vitamin K crosses the placenta, leaving the child with very low reserves. Because factors II, VII, IX, and X (clotting factors) are vitamin K–dependent, low levels of vitamin K can result in coagulation problems. Consequently, when a child presents with unexplained bruising, a coagulation evaluation may be warranted to determine whether there is an underlying disease.[16]
Lung and sinus
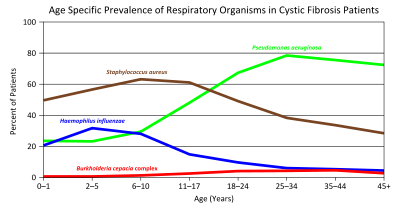 Respiratory infections in CF varies according to age.
Respiratory infections in CF varies according to age.
Green = Pseudomonas aeruginosa
Brown = Staphylococcus aureus
Blue = Haemophilus influenzae
Red = Burkholderia cepacia complexLung disease results from clogging of the airways due to mucus build-up, decreased mucociliary clearance and resulting inflammation.[17][18] Inflammation and infection will cause injury and structural changes to the lungs, leading to a variety of symptoms. In the early stages, incessant coughing, copious phlegm production, and decreased ability to exercise are common. Many of these symptoms occur when bacteria that normally inhabit the thick mucus grow out of control and cause pneumonia. In later stages, changes in the architecture of the lung such as pathology in the major airways (bronchiectasis) further exacerbate difficulties in breathing. Other symptoms include coughing up blood (hemoptysis), high blood pressure in the lung (pulmonary hypertension), heart failure, difficulties getting enough oxygen to the body (hypoxia), and respiratory failure requiring support with breathing masks such as bilevel positive airway pressure machines or ventilators.[19] Staphylococcus aureus, Haemophilus influenzae, and Pseudomonas aeruginosa are the three most common organisms causing lung infections in CF patients.[18] In addition to typical bacterial infections, people with CF more commonly develop other types of lung disease. Among these is allergic bronchopulmonary aspergillosis, in which the body's response to the common fungus Aspergillus fumigatus causes worsening of breathing problems. Another is infection with Mycobacterium avium complex (MAC), a group of bacteria related to tuberculosis, which can cause a lot of lung damage and does not respond to common antibiotics.[20]
Mucus in the paranasal sinuses is equally thick and may also cause blockage of the sinus passages, leading to infection. This may cause facial pain, fever, nasal drainage, and headaches. Individuals with CF may develop overgrowth of the nasal tissue (nasal polyps) due to inflammation from chronic sinus infections.[21] Recurrent sinonasal polyps can occur in as many as 10% to 25% of CF patients.[18] These polyps can block the nasal passages and increase breathing difficulties.[22][23]
Cardiorespiratory complications are the most common cause of death (~80%) in patients followed by most CF centers in the United States.[18]
Gastrointestinal
Prior to prenatal and newborn screening, cystic fibrosis was often diagnosed when a newborn infant failed to pass faeces (meconium). Meconium may completely block the intestines and cause serious illness. This condition, called meconium ileus, occurs in 5–10%[18][24] of newborns with CF. In addition, protrusion of internal rectal membranes (rectal prolapse) is more common, occurring in as many as 10% of children with CF,[18] and it is caused by increased fecal volume, malnutrition, and increased intra–abdominal pressure due to coughing.[25]
The thick mucus seen in the lungs has a counterpart in thickened secretions from the pancreas, an organ responsible for providing digestive juices which help break down food. These secretions block the exocrine movement of the digestive enzymes into the duodenum and result in irreversible damage to the pancreas, often with painful inflammation (pancreatitis).[26] The pancreatic ducts are totally plugged in more advanced cases, usually seen in older children or adolescents.[18] This causes atrophy of the exocrine glands and progressive fibrosis.[18]
The lack of digestive enzymes leads to difficulty absorbing nutrients with their subsequent excretion in the feces, a disorder known as malabsorption. Malabsorption leads to malnutrition and poor growth and development because of calorie loss. Resultant hypoproteinemia may be severe enough to cause generalized edema.[18] Individuals with CF also have difficulties absorbing the fat-soluble vitamins A, D, E, and K.
In addition to the pancreas problems, people with cystic fibrosis experience more heartburn, intestinal blockage by intussusception, and constipation.[27] Older individuals with CF may develop distal intestinal obstruction syndrome when thickened feces cause intestinal blockage.[28]
Exocrine pancreatic insufficiency occurs in the majority (85% to 90%) of patients with CF.[18] It is mainly associated with "severe" CFTR mutations, where both alleles are completely nonfunctional (e.g. ΔF508/ΔF508).[18] It occurs in 10% to 15% of patients with one "severe" and one "mild" CFTR mutation where there still is a little CFTR activity, or where there are two "mild" CFTR mutations.[18] In these milder cases, there is still sufficient pancreatic exocrine function so that enzyme supplementation is not required.[18] There are usually no other GI complications in pancreas-sufficient phenotypes, and in general, such individuals usually have excellent growth and development.[18] Despite this, idiopathic chronic pancreatitis can occur in a subset of pancreas-sufficient individuals with CF, and is associated with recurrent abdominal pain and life-threatening complications.[18]
Thickened secretions also may cause liver problems in patients with CF. Bile secreted by the liver to aid in digestion may block the bile ducts, leading to liver damage. Over time, this can lead to scarring and nodularity (cirrhosis). The liver fails to rid the blood of toxins and does not make important proteins such as those responsible for blood clotting.[29][30] Liver disease is the third most common cause of death associated with CF.[18]
Endocrine
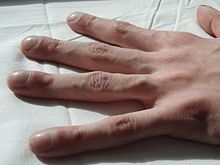
Clubbing in the fingers of a person with cystic fibrosis
The pancreas contains the islets of Langerhans, which are responsible for making insulin, a hormone that helps regulate blood glucose. Damage of the pancreas can lead to loss of the islet cells, leading to a type of diabetes that is unique to those with the disease.[31] This cystic fibrosis related diabetes (CFRD) shares characteristics that can be found in Type 1 and Type 2 diabetics, and is one of the principal non-pulmonary complications of CF.[32] Vitamin D is involved in calcium and phosphate regulation. Poor uptake of vitamin D from the diet because of malabsorption can lead to the bone disease osteoporosis in which weakened bones are more susceptible to fractures.[33] In addition, people with CF often develop clubbing of their fingers and toes due to the effects of chronic illness and low oxygen in their tissues.[34][35]
Infertility
Infertility affects both men and women. At least 97% of men with cystic fibrosis are infertile, but not sterile and can have children with assisted reproductive techniques.[36] These men make normal sperm but are missing the tube (vas deferens), which connects the testes to the ejaculatory ducts of the penis.[37] Many men found to have congenital absence of the vas deferens during evaluation for infertility have a mild, previously undiagnosed form of CF.[38] Some women have fertility difficulties due to thickened cervical mucus or malnutrition. In severe cases, malnutrition disrupts ovulation and causes amenorrhea.[39]
Cause
CF is caused by a mutation in the gene cystic fibrosis transmembrane conductance regulator (CFTR). The most common mutation, ΔF508, is a deletion (Δ) of three nucleotides[40] that results in a loss of the amino acid phenylalanine (F) at the 508th position on the protein. This mutation accounts for two-thirds (66-70%[18]) of CF cases worldwide and 90% of cases in the United States; however, there are over 1500 other mutations that can produce CF.[41] Although most people have two working copies (alleles) of the CFTR gene, only one is needed to prevent cystic fibrosis. CF develops when neither allele can produce a functional CFTR protein. Thus, CF is considered an autosomal recessive disease.
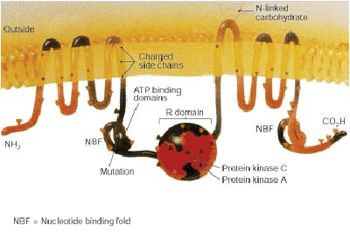
The CFTR gene, found at the q31.2 locus of chromosome 7, is 230,000 base pairs long, and creates a protein that is 1,480 amino acids long. Structurally, CFTR is a type of gene known as an ABC gene.[19] The product of this gene (the CFTR) is a chloride ion channel important in creating sweat, digestive juices and mucus. This protein possesses two ATP-hydrolyzing domains which allows the protein to use energy in the form of ATP. It also contains two domains comprising 6 alpha helices apiece, which allow the protein to cross the cell membrane. A regulatory binding site on the protein allows activation by phosphorylation, mainly by cAMP-dependent protein kinase.[19] The carboxyl terminal of the protein is anchored to the cytoskeleton by a PDZ domain interaction.[42]
In addition, there is increasing evidence that genetic modifiers besides CFTR modulate the frequency and severity of the disease. One example is mannan-binding lectin, which is involved in innate immunity by facilitating phagocytosis of microorganisms. Polymorphisms in one or both mannan-binding lectin alleles that result in lower circulating levels of the protein are associated with a threefold higher risk of end-stage lung disease, as well as an increased burden of chronic bacterial infections.[18]
Pathophysiology
There are several mechanisms by which mutations cause problems with the CFTR protein. ΔF508, for instance, creates a protein that does not fold normally and is degraded by the cell. Several different mutations result in proteins that are too short because production is ended prematurely. Less common mutations produce proteins that do not use energy normally, do not allow chloride, iodide and thiocyanate to cross the membrane appropriately,[43] or are degraded at a faster rate than normal. Mutations may also lead to fewer copies of the CFTR protein being produced.[19]
The protein created by this gene is anchored to the outer membrane of cells in the sweat glands, lungs, pancreas, and other affected organs. The protein spans this membrane and acts as a channel connecting the inner part of the cell (cytoplasm) to the surrounding fluid. This channel is primarily responsible for controlling the movement of halogens from inside to outside of the cell; however, in the sweat ducts it facilitates the movement of chloride from the sweat into the cytoplasm. When the CFTR protein does not work, chloride and thiocyanate[44] are trapped inside the cells in the airway and outside in the skin. Then hypothiocyanite, OSCN, cannot be produced by immune defense system.[45][46] Because chloride is negatively charged, this creates a difference in the electrical potential inside and outside the cell causing cations to cross into the cell. Sodium is the most common cation in the extracellular space and the combination of sodium and chloride creates the salt, which is lost in high amounts in the sweat of individuals with CF. This lost salt forms the basis for the sweat test.[19]
How this malfunction of cells in cystic fibrosis causes the clinical manifestations is not well understood. One theory suggests that the lack of halogen and pseudohalogen (mainly, chloride, iodide and thiocyanate) exodus through the CFTR protein leads to the accumulation of more viscous, nutrient-rich mucus in the lungs that allows bacteria to hide from the body's immune system. Another theory proposes that the CFTR protein failure leads to a paradoxical increase in sodium and chloride uptake, which, by leading to increased water reabsorption, creates dehydrated and thick mucus. Yet another theory focuses on abnormal chloride movement out of the cell, which also leads to dehydration of mucus, pancreatic secretions, biliary secretions, etc. These theories all support the observation that the majority of the damage in CF is due to blockage of the narrow passages of affected organs with thickened secretions. These blockages lead to remodeling and infection in the lung, damage by accumulated digestive enzymes in the pancreas, blockage of the intestines by thick faeces, etc.[19]
Chronic infections
The lungs of individuals with cystic fibrosis are colonized and infected by bacteria from an early age. These bacteria, which often spread among individuals with CF, thrive in the altered mucus, which collects in the small airways of the lungs. This mucus leads to the formation of bacterial microenvironments known as biofilms that are difficult for immune cells and antibiotics to penetrate. Viscous secretions and persistent respiratory infections repeatedly damage the lung by gradually remodeling the airways which makes infection even more difficult to eradicate.[47]
Over time, both the types of bacteria and their individual characteristics change in individuals with CF. In the initial stage, common bacteria such as Staphylococcus aureus and Hemophilus influenzae colonize and infect the lungs.[18] Eventually, Pseudomonas aeruginosa (and sometimes Burkholderia cepacia) dominates. By 18 years of age, 80% of patients with classic CF harbor P. aeruginosa, and 3.5% harbor B. cepacia.[18] Once within the lungs, these bacteria adapt to the environment and develop resistance to commonly used antibiotics. Pseudomonas can develop special characteristics that allow the formation of large colonies, known as "mucoid" Pseudomonas, which are rarely seen in people that do not have CF.[47]
One way in which infection has spread is by passage between different individuals with CF.[48] In the past, people with CF often participated in summer "CF Camps" and other recreational gatherings.[49][50] Hospitals grouped patients with CF into common areas and routine equipment (such as nebulizers)[51] was not sterilized between individual patients.[52] This led to transmission of more dangerous strains of bacteria among groups of patients. As a result, individuals with CF are routinely isolated from one another in the healthcare setting and healthcare providers are encouraged to wear gowns and gloves when examining patients with CF to limit the spread of virulent bacterial strains.[53]
CF patients may also have their airways chronically colonized by filamentous fungi (such as Aspergillus fumigatus, Scedosporium apiospermum, Aspergillus terreus) and/or yeasts (such as Candida albicans); other filamentous fungi less commonly isolated include Aspergillus flavus and Aspergillus nidulans (occur transiently in CF respiratory secretions), and Exophiala dermatitidis and Scedosporium prolificans (chronic airway-colonizers); some filamentous fungi like Penicillium emersonii and Acrophialophora fusispora are encountered in patients almost exclusively in the context of CF.[54] Defective mucociliary clearance characterizing CF is associated with local immunological disorders. In addition, the prolonged therapy with antibiotics and the use of corticosteroid treatments may also facilitate fungal growth. Although the clinical relevance of the fungal airway colonization is still a matter of debate, filamentous fungi may contribute to the local inflammatory response, and therefore to the progressive deterioration of the lung function, as often happens with allergic broncho-pulmonary aspergillosis (ABPA) - the most common fungal disease in the context of CF, involving a Th2-driven immune response to Aspergillus.[54][55]
Diagnosis and monitoring
The location of the CFTR gene on chromosome 7
Cystic fibrosis may be diagnosed by many different methods including newborn screening, sweat testing, and genetic testing. As of 2006 in the United States, 10 percent of cases are diagnosed shortly after birth as part of newborn screening programs. The newborn screen initially measures for raised blood concentration of immunoreactive trypsinogen.[56] Infants with an abnormal newborn screen need a sweat test in order to confirm the CF diagnosis. In many cases, a parent makes the diagnosis because the infant tastes salty.[18] Trypsinogen levels can be increased in individuals who have a single mutated copy of the CFTR gene (carriers) or, in rare instances, in individuals with two normal copies of the CFTR gene. Due to these false positives, CF screening in newborns can be controversial.[57][58] Most states and countries do not screen for CF routinely at birth. Therefore, most individuals are diagnosed after symptoms (e.g. sinopulmonary disease and GI manifestations[18]) prompt an evaluation for cystic fibrosis. The most commonly used form of testing is the sweat test. Sweat-testing involves application of a medication that stimulates sweating (pilocarpine). In order to deliver the medication through the skin, iontophoresis is used to, whereby one electrode is placed onto the applied medication and an electric current is passed to a separate electrode on the skin. The resultant sweat is then collected on filter paper or in a capillary tube and analyzed for abnormal amounts of sodium and chloride. People with CF have increased amounts of sodium and chloride in their sweat. In opposite, people with CF have less thiocyanate and hypothiocyanite in their saliva (Minarowski[59] et al.) and mucus (Banfi et al.). CF can also be diagnosed by identification of mutations in the CFTR gene.[60]
People with CF may be listed in a disease registry that allows researchers and doctors to track health results and identify candidates for clinical trials.[61]
Prenatal
Couples who are pregnant or who are planning a pregnancy can themselves be tested for CFTR gene mutations to determine the degree of risk that their child will be born with cystic fibrosis. Testing is typically performed first on one or both parents and, if the risk of CF is found to be high, testing on the fetus can then be performed. The American College of Obstetricians and Gynecologists (ACOG) recommends testing for couples who have a personal or close family history of CF, and they recommend that carrier testing be offered to all Caucasian couples and be made available to couples of other ethnic backgrounds.[62]
Because development of CF in the fetus requires each parent to pass on a mutated copy of the CFTR gene and because CF testing is expensive, testing is often performed initially on one parent. If that parent is found to be a carrier of a CFTR gene mutation, the other parent is then tested to calculate the risk that their children will have CF. CF can result from more than a thousand different mutations, and as of 2006 it is not possible to test for each one. Testing analyzes the blood for the most common mutations such as ΔF508—most commercially available tests look for 32 or fewer different mutations. If a family has a known uncommon mutation, specific screening for that mutation can be performed. Because not all known mutations are found on current tests, a negative screen does not guarantee that a child will not have CF.[63]
During pregnancy, testing can be performed on the placenta (chorionic villus sampling) or the fluid around the fetus (amniocentesis). However, chorionic villus sampling has a risk of fetal death of 1 in 100 and amniocentesis of 1 in 200;[64] a recent study has indicated this may be much lower, approximately 1 in 1,600.[65]
Economically, for carrier couples of cystic fibrosis, when comparing preimplantation genetic diagnosis (PGD) with natural conception (NC) followed by prenatal testing and abortion of affected pregnancies, PGD provides net economic benefits up to a maternal age of approximately 40 years, after which NC, prenatal testing and abortion has higher economic benefit.[66]
Management
While there are no cures for cystic fibrosis there are several treatment methods. The management of cystic fibrosis has improved significantly over the past 70 years. While infants born with cystic fibrosis 70 years ago would have been unlikely to live beyond their first year, infants today are likely to live well into adulthood. Recent advances in the treatment of cystic fibrosis have meant that an individual with cystic fibrosis can live a fuller life less encumbered by their condition. The cornerstones of management are proactive treatment of airway infection, and encouragement of good nutrition and an active lifestyle. Management of cystic fibrosis continues throughout a patient's life, and is aimed at maximizing organ function, and therefore quality of life. At best, current treatments delay the decline in organ function. Because of the wide variation in disease symptoms treatment typically occurs at specialist multidisciplinary centers, and is tailored to the individual. Targets for therapy are the lungs, gastrointestinal tract (including pancreatic enzyme supplements), the reproductive organs (including assisted reproductive technology (ART)) and psychological support.[56]
The most consistent aspect of therapy in cystic fibrosis is limiting and treating the lung damage caused by thick mucus and infection, with the goal of maintaining quality of life. Intravenous, inhaled, and oral antibiotics are used to treat chronic and acute infections. Mechanical devices and inhalation medications are used to alter and clear the thickened mucus. These therapies, while effective, can be extremely time-consuming for the patient. One of the most important battles that CF patients face is finding the time to comply with prescribed treatments while balancing a normal life.
In addition, therapies such as transplantation and gene therapy aim to cure some of the effects of cystic fibrosis. Gene therapy aims to introduce normal CFTR to airway. Theoretically this process should be simple as the airway is easily accessible and there is only a single gene defect to correct. There are two CFTR gene introduction mechanisms involved, the first use of a viral vector (adenovirus, adeno-associated virus or retro virus) and secondly the use of liposome. However there are some problems associated with these methods involving efficiency (liposomes insufficient protein) and delivery (virus provokes an immune response).
Antibiotics
Many CF patients are on one or more antibiotics at all times, even when they are considered healthy, in order to prophylactically suppress infection. Antibiotics are absolutely necessary whenever pneumonia is suspected or there has been a noticeable decline in lung function, and are usually chosen based on the results of a sputum analysis and the patient's past response. This prolonged therapy often necessitates hospitalization and insertion of a more permanent IV such as a peripherally inserted central catheter (PICC line) or Port-a-Cath. Inhaled therapy with antibiotics such as tobramycin, colistin, and aztreonam is often given for months at a time in order to improve lung function by impeding the growth of colonized bacteria.[67][68][69] Oral antibiotics such as ciprofloxacin or azithromycin are given to help prevent infection or to control ongoing infection.[70] The aminoglycoside antibiotics (e.g. tobramycin) used can cause hearing loss, damage to the balance system in the inner ear or kidney problems with long-term use.[71] In order to prevent these side-effects, the amount of antibiotics in the blood are routinely measured and adjusted accordingly.
Other treatments for lung disease
Several mechanical techniques are used to dislodge sputum and encourage its expectoration. In the hospital setting, chest physiotherapy (CPT) is utilized; a respiratory therapist percusses an individual's chest with his or her hands several times a day, to loosen up secretions. Devices that recreate this percussive therapy include the ThAIRapy Vest and the intrapulmonary percussive ventilator (IPV). Newer methods such as Biphasic Cuirass Ventilation, and associated clearance mode available in such devices, integrate a cough assistance phase, as well as a vibration phase for dislodging secretions. These are portable and adapted for home use.[72]
Aerosolized medications that help loosen secretions include dornase alfa and hypertonic saline.[73] Dornase is a recombinant human deoxyribonuclease, which breaks down DNA in the sputum, thus decreasing its viscosity.[74] Denufosol is an investigational drug that opens an alternative chloride channel, helping to liquefy mucus.[75]
As lung disease worsens, mechanical breathing support may become necessary. Individuals with CF may need to wear special masks at night that help push air into their lungs. These machines, known as bilevel positive airway pressure (BiPAP) ventilators, help prevent low blood oxygen levels during sleep. BiPAP may also be used during physical therapy to improve sputum clearance.[76] During severe illness, a tube may be placed in the throat (a procedure known as a tracheostomy) to enable breathing supported by a ventilator.
For children living with CF, preliminary studies show pediatric massage therapy may improve patients and their families quality of life, though more rigorous studies need to be done.[77]
Transplantation
Lung transplantation often becomes necessary for individuals with cystic fibrosis as lung function and exercise tolerance declines. Although single lung transplantation is possible in other diseases, individuals with CF must have both lungs replaced because the remaining lung might contain bacteria that could infect the transplanted lung. A pancreatic or liver transplant may be performed at the same time in order to alleviate liver disease and/or diabetes.[78] Lung transplantation is considered when lung function declines to the point where assistance from mechanical devices is required or patient survival is threatened.[79]
Treatment of other aspects
Newborns with CF typically require surgery, whereas adults with distal intestinal obstruction syndrome typically do not. Treatment of pancreatic insufficiency by replacement of missing digestive enzymes allows the duodenum to properly absorb nutrients and vitamins that would otherwise be lost in the feces.So far, no large-scale research involving the incidence of atherosclerosis and coronary heart disease in adults with cystic fibrosis has been conducted. This is likely due to the fact that the vast majority of people with cystic fibrosis do not live long enough to develop clinically significant atherosclerosis or coronary heart disease.
Diabetes is the most common non-pulmonary complication of CF. It mixes features of type 1 and type 2 diabetes, and is recognized as a distinct entity, cystic fibrosis-related diabetes (CFRD).[80][81] While oral anti-diabetic drugs are sometimes used, the only recommended treatment is the use of insulin injections or an insulin pump,[82] and, unlike in type 1 and 2 diabetes, dietary restrictions are not recommended.[80]
Development of osteoporosis can be prevented by increased intake of vitamin D and calcium, and can be treated by bisphosphonates, although adverse effects can be an issue.[83] Poor growth may be avoided by insertion of a feeding tube for increasing calories through supplemental feeds or by administration of injected growth hormone.[84]
Sinus infections are treated by prolonged courses of antibiotics. The development of nasal polyps or other chronic changes within the nasal passages may severely limit airflow through the nose, and over time reduce the patient's sense of smell. Sinus surgery is often used to alleviate nasal obstruction and to limit further infections. Nasal steroids such as fluticasone are used to decrease nasal inflammation.[85] Female infertility may be overcome by assisted reproduction technology, particularly embryo transfer techniques. Male infertility caused by absence of the vas deferens may be overcome with testicular sperm extraction (TEST), collecting sperm cells directly from the testicles. If the collected sample contains too few sperm cells to likely have a spontaneous fertilization, intracytoplasmic sperm injection can be performed.[86] Third party reproduction is also a possibility for women with CF.
Quality of life
Chronic illnesses are very difficult to manage. Cystic fibrosis (CF) is a chronic illness that affects the “digestive and respiratory tracts resulting in generalized malnutrition and chronic respiratory infections”. [87]The thick secretions clog the airways in the lungs, which often cause inflammation and severe lung infections.[88] Therefore, mucus makes it challenging to breathe. If it is compromised, it will affect the quality of life of someone with CF, and his or her ability to complete such tasks as everyday chores. It is important for CF patients to understand the detrimental relationship that chronic illnesses place on the quality of life. According to Schmitz and Goldbeck (2006), the fact that Cystic Fibrosis significantly increases emotional stress on both the individual and the family, “and the necessary time-consuming daily treatment routine may have further negative effects on quality of life (QOL)”. [89] However, Havermans and colleagues (2006) have shown that young outpatients with CF that have participated in the CFQ-R (Cystic Fibrosis Questionnaire-Revised) “rated some QOL domains higher than did their parents”.[90] Consequently, outpatients with CF have a more positive outlook for themselves. Furthermore, there are many ways to improve the QOL in CF patients. Exercise is promoted to increase lung function. The fact of integrating an exercise regime into the CF patient’s daily routine can significantly improve the quality of life. [91] There is no definitive cure for Cystic Fibrosis. However, there are diverse medications used such as, mucolytics, bronchodilators, steroids and antibiotics that have the purpose of loosening mucus, expanding airways, decreasing inflammation and fighting lung infections. [92]
Prognosis
The improved prognosis of cystic fibrosis, combined with earlier diagnosis through screening, has already started to result in a change in attitude. Many factors will influence the prognosis of a person with cystic fibrosis. These factors include treatment compliance, efficacy of treatment, and access to health care.
Life expectancy for people with CF depends largely upon access to health care. In 1959, the median age of survival of children with cystic fibrosis was six months. In the United States, the life expectancy for infants born in 2008 with CF is 37.4 years, based upon data compiled by the Cystic Fibrosis Foundation.[93] The median survival age in Canada has increased from 24 in 1982 to 47.7 in 2007, based on data compiled by the Canadian Cystic Fibrosis Foundation.[94]
The U.S. Cystic Fibrosis Foundation compiles lifestyle information about American adults with CF. In 2008, the foundation reported that 92% had graduated from high school and 66% had at least some college education. Employment data revealed 15% of adults were disabled and 7% were unemployed. Marital information showed that 54.8% of adults were single and 40.1% were married or living with a partner. In 2008, 240 American women with CF were pregnant.[95]
Epidemiology
Mutation Frequency
worldwide[96]ΔF508 66%-70%[18] G542X 2.4% G551D 1.6% N1303K 1.3% W1282X 1.2% All others 27.5% Cystic fibrosis is the most common life-limiting autosomal recessive disease among people of European heritage.[97] In the United States, approximately 30,000 individuals have CF; most are diagnosed by six months of age. Canada has approximately 3,000 citizens with CF. Approximately 1 in 25 people of European descent, and one in 30 of Caucasian Americans,[98] is a carrier of a cystic fibrosis mutation. Although CF is less common in these groups, approximately 1 in 46 Hispanics, 1 in 65 Africans and 1 in 90 Asians carry at least one abnormal CFTR gene.[99][100]
Although technically a rare disease, cystic fibrosis is ranked as one of the most widespread life-shortening genetic diseases. It is most common among nations in the Western world. An exception is Finland, where only one in 80 people carry a CF mutation.[101] In the United States, 1 in 4,000 children are born with CF.[102] In 1997, about 1 in 3,300 caucasian children in the United States was born with cystic fibrosis. In contrast, only 1 in 15,000 African American children suffered from cystic fibrosis, and in Asian Americans the rate was even lower at 1 in 32,000.[103]
Cystic fibrosis is diagnosed in males and females equally. For reasons that remain unclear, data has shown that males tend to have a longer life expectancy than females,[104][105] however recent studies suggest this gender gap may no longer exist perhaps due to improvements in health care facilities,[106][107] while a recent study from Ireland identified a link between the female hormone oestrogen and worse outcomes in CF.[108]
The distribution of CF alleles varies among populations. The frequency of ΔF508 carriers has been estimated to be 1:200 in northern Sweden, 1:143 in Lithuanians, and 1:38 in Denmark. No ΔF508 carriers were found among 171 Finns and 151 Saami people.[109] ΔF508 does occur in Finland, but it is a minority allele there. Cystic fibrosis is known to occur in only 20 families (pedigrees) in Finland.[110]
Theories about prevalence
The ΔF508 mutation is estimated to be up to 52,000 years old.[111] Numerous hypotheses have been advanced as to why such a lethal mutation has persisted and spread in the human population. Other common autosomal recessive diseases such as sickle-cell anemia have been found to protect carriers from other diseases, a concept known as heterozygote advantage. Resistance to the following have all been proposed as possible sources of heterozygote advantage:
- Cholera: With the discovery that cholera toxin requires normal host CFTR proteins to function properly, it was hypothesized that carriers of mutant CFTR genes benefited from resistance to cholera and other causes of diarrhea.[112] Further studies have not confirmed this hypothesis.[113][114]
- Typhoid: Normal CFTR proteins are also essential for the entry of Salmonella typhi into cells,[115] suggesting that carriers of mutant CFTR genes might be resistant to typhoid fever. No in vivo study has yet confirmed this. In both cases, the low level of cystic fibrosis outside of Europe, in places where both cholera and typhoid fever are endemic, is not immediately explicable.
- Diarrhea: It has also been hypothesized that the prevalence of CF in Europe might be connected with the development of cattle domestication. In this hypothesis, carriers of a single mutant CFTR chromosome had some protection from diarrhea caused by lactose intolerance, prior to the appearance of the mutations that created lactose tolerance.[116]
- Tuberculosis: Another possible explanation is that carriers of the gene could have some resistance to TB.[117][118]
History
 National Library of Medicine photo of Dorothy Hansine Andersen. Andersen first described cystic fibrosis in 1938.
National Library of Medicine photo of Dorothy Hansine Andersen. Andersen first described cystic fibrosis in 1938.
It is supposed that CF appeared about 3,000 BC as a cause of migration of peoples, gene mutations, and new conditions in nourishment.[119] Although the entire clinical spectrum of CF was not recognized until the 1930s, certain aspects of CF were identified much earlier. Indeed, literature[specify] from Germany and Switzerland in the 18th century warned Wehe dem Kind, das beim Kuß auf die Stirn salzig schmekt, er ist verhext und muss bald sterbe or "Woe is the child who tastes salty from a kiss on the brow, for he is cursed, and soon must die," recognizing the association between the salt loss in CF and illness.[119]
In the 19th century, Carl von Rokitansky described a case of fetal death with meconium peritonitis, a complication of meconium ileus associated with cystic fibrosis. Meconium ileus was first described in 1905 by Karl Landsteiner.[119] In 1936, Guido Fanconi published a paper describing a connection between celiac disease, cystic fibrosis of the pancreas, and bronchiectasis.[120]
In 1938 Dorothy Hansine Andersen published an article, "Cystic Fibrosis of the Pancreas and Its Relation to Celiac Disease: a Clinical and Pathological Study," in the American Journal of Diseases of Children. She was the first to describe the characteristic cystic fibrosis of the pancreas and to correlate it with the lung and intestinal disease prominent in CF.[2] She also first hypothesized that CF was a recessive disease and first used pancreatic enzyme replacement to treat affected children. In 1952 Paul di Sant' Agnese discovered abnormalities in sweat electrolytes; a sweat test was developed and improved over the next decade.[121]
In 1988 the first mutation for CF, ΔF508 was discovered by Francis Collins, Lap-Chee Tsui and John R. Riordan on the seventh chromosome. Subsequent research has found over 1,000 different mutations that cause CF.
Because mutations in the CFTR gene are typically small, classical genetics techniques had been unable to accurately pinpoint the mutated gene.[122] Using protein markers, gene-linkage studies were able to map the mutation to chromosome 7. Chromosome-walking and -jumping techniques were then used to identify and sequence the gene.[123] In 1989 Lap-Chee Tsui led a team of researchers at the Hospital for Sick Children in Toronto that discovered the gene responsible for CF. Cystic fibrosis represents the first genetic disorder elucidated strictly by the process of reverse genetics.
Research
Gene therapy
Gene therapy has been explored as a potential cure for cystic fibrosis. Ideally, gene therapy attempts to place a normal copy of the CFTR gene into affected cells. Transferring the normal CFTR gene into the affected epithelium cells would result in the production of functional CFTR in all target cells, without adverse reactions or an inflammation response. Studies have shown that to prevent the lung manifestations of cystic fibrosis, only 5–10% the normal amount of CFTR gene expression is needed.[124] Multiple approaches have been tested for gene transfer, such as liposomes and viral vectors in animal models and clinical trials. However, both methods were found to be relatively inefficient treatment options.[125] The main reason is that very few cells take up the vector and express the gene, so the treatment has little effect. Additionally, problems have been noted in cDNA recombination, such that the gene introduced by the treatment is rendered unusable.[126] Gene therapy has made massive advances in the way that cystic fibrosis has been treated throughout the world[citation needed]. With the help of the Cystic Fibrosis Trust, which has a league of highly professional gene therapists, both somatic and Adeno-associated viral vector have made advances. The Adenoviridae, or more commonly known as the cold virus, is genetically altered, allowing the CFTR gene to enter lung cells. They are currently stage 3 clinical trial, and following the success of these trials, the treatment can become readily available and valid in the eyes of the medical world[citation needed].
Small molecules
A number of small molecules that aim at compensating various mutations of the CFTR gene are under development. One approach is to develop drugs that will get the ribosome to overcome the stop codon and synthesize a full-length CFTR protein. About 10% of CF result from a premature stop codon in the DNA, leading to early termination of protein synthesis and truncated proteins. These drugs target nonsense mutations such as G542X, which consists of the amino acid glycin in position 542 being replaced by a stop codon. Aminoglycoside antibiotics interfere with DNA synthesis and error-correction. In some cases, they can cause the cell to overcome the stop codon, insert a random amino acid, and express a full-length protein.[127] The aminoglycoside gentamicin has been used to treat lung cells from CF patients in the laboratory to induce the cells to grown full-length proteins.[128] Another drug targeting nonsense mutations is ataluren, which is undergoing Phase III clinical trials as of October 2011[update].[129]
Ivacaftor, also in Phase III trials, targets the mutation G551D (glycin in position 551 is substituted with aspartic acid). VX-807 aims at F508del (phenylalanin in position 508 is missing).[130]
See also
- List of people diagnosed with cystic fibrosis
- 65 Redroses
References
- ^ Yankas JR, et al. (2004). "Cystic fibrosis adult care consensus conference report". Chest 125: 1-39.
- ^ a b Andersen DH. Cystic fibrosis of the pancreas and its relation to celiac disease: a clinical and pathological study. Am J Dis Child 1938; 56:344-399
- ^ Ratjen F, Doring G; Cystic fibrosis. Lancet. 2003 Feb 22;361(9358):681-9.
- ^ Patient UK website - Cystic Fibrosis
- ^ Scientists make major cystic fibrosis breakthrough | BreakingNews.ie
- ^ We have the highest rate of cystic fibrosis in the world - Health, Frontpage - Independent.ie
- ^ RTÉ Television - The Afternoon Show
- ^ Kliegman, Robert; Richard M Kliegman (2006). Nelson essentials of pediatrics. St. Louis, Mo: Elsevier Saunders. ISBN 0-8089-2325-0.
- ^ Quinton PM (June 2007). "Cystic fibrosis: lessons from the sweat gland". Physiology (Bethesda) 22 (3): 212–25. doi:10.1152/physiol.00041.2006. PMID 17557942. http://nips.physiology.org/cgi/pmidlookup?view=long&pmid=17557942.
- ^ a b Hardin DS (August 2004). "GH improves growth and clinical status in children with cystic fibrosis -- a review of published studies". Eur. J. Endocrinol. 151 Suppl 1: S81–5. doi:10.1530/eje.0.151S081. PMID 15339250. http://eje-online.org/cgi/pmidlookup?view=long&pmid=15339250.
- ^ a b De Lisle RC (September 2009). "Pass the bicarb: the importance of HCO3- for mucin release". J. Clin. Invest. 119 (9): 2535–7. doi:10.1172/JCI40598. PMC 2735941. PMID 19726878. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2735941.
- ^ O'Malley CA (May 2009). "Infection control in cystic fibrosis: cohorting, cross-contamination, and the respiratory therapist". Respir Care 54 (5): 641–57. doi:10.4187/aarc0446. PMID 19393108. http://www.rcjournal.com/contents/05.09/05.09.0641.pdf.
- ^ Makker K, Agarwal A, Sharma R (April 2009). "Oxidative stress & male infertility". Indian J. Med. Res. 129 (4): 357–67. PMID 19535829. http://www.icmr.nic.in/ijmr/2009/april/0403.pdf.
- ^ Blackman SM, Deering-Brose R, McWilliams R, et al. (October 2006). "Relative contribution of genetic and nongenetic modifiers to intestinal obstruction in cystic fibrosis". Gastroenterology 131 (4): 1030–9. doi:10.1053/j.gastro.2006.07.016. PMC 1764617. PMID 17030173. http://linkinghub.elsevier.com/retrieve/pii/S0016-5085(06)01659-3.
- ^ Ratjen FA (May 2009). "Cystic fibrosis: pathogenesis and future treatment strategies". Respir Care 54 (5): 595–605. doi:10.4187/aarc0427. PMID 19393104. http://www.rcjournal.com/contents/05.09/05.09.0595.pdf.
- ^ Reaves J, Wallace G. Unexplained bruising: weighing the pros and cons of possible causes. Consultant for Pediatricians. 2010;9:201-202.
- ^ Flume PA, Mogayzel Jr PJ, Robinson KA, et al. (March 2010). "Cystic Fibrosis Pulmonary Guidelines: Pulmonary Complications: Hemoptysis and Pneumothorax". Am J Respir Crit Care Med 182 (3): 298. doi:10.1164/rccm.201002-0157OC. PMID 20299528. http://ajrccm.atsjournals.org/cgi/pmidlookup?view=long&pmid=20299528.[dead link]
- ^ a b c d e f g h i j k l m n o p q r s t u v w Mitchell, Richard Sheppard; Kumar, Vinay; Robbins, Stanley L.; Abbas, Abul K.; Fausto, Nelson (2007). Robbins basic pathology. Saunders/Elsevier. ISBN 1-4160-2973-7.
- ^ a b c d e f Rowe SM, Miller S, Sorscher EJ (May 2005). "Cystic fibrosis". The New England Journal of Medicine 352 (19): 1992–2001. doi:10.1056/NEJMra043184. PMID 15888700.
- ^ Girón RM, Domingo D, Buendía B, Antón E, Ruiz-Velasco LM, Ancochea J (October 2005). "Nontuberculous mycobacteria in patients with cystic fibrosis" (in Spanish; Castilian). Arch. Bronconeumol. 41 (10): 560–5. doi:10.1016/S1579-2129(06)60283-8. PMID 16266669. http://www.elsevier.es/revistas/0300-2896/41/560.
- ^ Franco LP, Camargos PA, Becker HM, Guimarães RE (December 2009). "Nasal endoscopic evaluation of children and adolescents with cystic fibrosis". Braz J Otorhinolaryngol 75 (6): 806–13. PMID 20209279. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1808-86942009000600006&lng=en&nrm=iso&tlng=en.
- ^ Maldonado M, Martínez A, Alobid I, Mullol J (December 2004). "The antrochoanal polyp". Rhinology 42 (4): 178–82. PMID 15626248.
- ^ Ramsey B, Richardson MA (September 1992). "Impact of sinusitis in cystic fibrosis". J. Allergy Clin. Immunol. 90 (3 Pt 2): 547–52. doi:10.1016/0091-6749(92)90183-3. PMID 1527348.
- ^ Eggermont E, De Boeck K (October 1991). "Small-intestinal abnormalities in cystic fibrosis patients". Eur. J. Pediatr. 150 (12): 824–8. doi:10.1007/BF01954999. PMID 1743211.
- ^ Kulczycki LL, Shwachman H (August 1958). "Studies in cystic fibrosis of the pancreas; occurrence of rectal prolapse". N. Engl. J. Med. 259 (9): 409–12. doi:10.1056/NEJM195808282590901. PMID 13578072.
- ^ Cohn JA, Friedman KJ, Noone PG, Knowles MR, Silverman LM, Jowell PS (September 1998). "Relation between mutations of the cystic fibrosis gene and idiopathic pancreatitis". N. Engl. J. Med. 339 (10): 653–8. doi:10.1056/NEJM199809033391002. PMID 9725922.
- ^ Malfroot A, Dab I (November 1991). "New insights on gastro-oesophageal reflux in cystic fibrosis by longitudinal follow up". Arch. Dis. Child. 66 (11): 1339–45. doi:10.1136/adc.66.11.1339. PMC 1793275. PMID 1755649. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1793275.
- ^ Khoshoo V, Udall JN (February 1994). "Meconium ileus equivalent in children and adults". Am. J. Gastroenterol. 89 (2): 153–7. PMID 8304294.
- ^ Williams SG, Westaby D, Tanner MS, Mowat AP (October 1992). "Liver and biliary problems in cystic fibrosis". Br. Med. Bull. 48 (4): 877–92. PMID 1458306.
- ^ Colombo C, Russo MC, Zazzeron L, Romano G (July 2006). "Liver disease in cystic fibrosis". J. Pediatr. Gastroenterol. Nutr. 43 Suppl 1: S49–55. doi:10.1097/01.mpg.0000226390.02355.52. PMID 16819402.
- ^ Moran A, Pyzdrowski KL, Weinreb J, et al. (August 1994). "Insulin sensitivity in cystic fibrosis". Diabetes 43 (8): 1020–6. doi:10.2337/diabetes.43.8.1020. PMID 8039595.
- ^ Alves Cde A, Aguiar RA, Alves AC, Santana MA (April 2007). "Diabetes mellitus in patients with cystic fibrosis". J Bras Pneumol 33 (2): 213–21. doi:10.1590/S1806-37132007000200017. PMID 17724542.
- ^ Haworth CS, Selby PL, Webb AK, et al. (November 1999). "Low bone mineral density in adults with cystic fibrosis". Thorax 54 (11): 961–7. doi:10.1136/thx.54.11.961. PMC 1745400. PMID 10525552. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1745400.
- ^ Vandemergel X, Decaux G (April 2003). "[Review on hypertrophic osteoarthropathy and digital clubbing]" (in French). Rev Med Brux 24 (2): 88–94. PMID 12806875.
- ^ Pitts-Tucker TJ, Miller MG, Littlewood JM (June 1986). "Finger clubbing in cystic fibrosis". Arch. Dis. Child. 61 (6): 576–9. doi:10.1136/adc.61.6.576. PMC 1777828. PMID 3488032. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1777828.
- ^ McCallum TJ, Milunsky JM, Cunningham DL, Harris DH, Maher TA, Oates RD (October 2000). "Fertility in men with cystic fibrosis: an update on current surgical practices and outcomes". Chest 118 (4): 1059–62. doi:10.1378/chest.118.4.1059. PMID 11035677.
- ^ Dodge JA (September 1995). "Male fertility in cystic fibrosis". Lancet 346 (8975): 587–8. doi:10.1016/S0140-6736(95)91431-5. PMID 7650999.
- ^ Augarten A, Yahav Y, Kerem BS, et al. (November 1994). "Congenital bilateral absence of vas deferens in the absence of cystic fibrosis". Lancet 344 (8935): 1473–4. doi:10.1016/S0140-6736(94)90292-5. PMID 7968122.
- ^ Gilljam M, Antoniou M, Shin J, Dupuis A, Corey M, Tullis DE (July 2000). "Pregnancy in cystic fibrosis. Fetal and maternal outcome". Chest 118 (1): 85–91. doi:10.1378/chest.118.1.85. PMID 10893364.
- ^ http://www.science.ca/scientists/scientistprofile.php?pID=19
- ^ Bobadilla JL, Macek M, Fine JP, Farrell PM (June 2002). "Cystic fibrosis: a worldwide analysis of CFTR mutations--correlation with incidence data and application to screening". Hum. Mutat. 19 (6): 575–606. doi:10.1002/humu.10041. PMID 12007216.
- ^ Short DB, Trotter KW, Reczek D, et al. (July 1998). "An apical PDZ protein anchors the cystic fibrosis transmembrane conductance regulator to the cytoskeleton". J. Biol. Chem. 273 (31): 19797–801. doi:10.1074/jbc.273.31.19797. PMID 9677412.
- ^ Childers M, Eckel G, Himmel A, Caldwell J (2007). "A new model of cystic fibrosis pathology: lack of transport of glutathione and its thiocyanate conjugates". Medical Hypotheses 68 (1): 101–12. doi:10.1016/j.mehy.2006.06.020. PMID 16934416.
- ^ Xu Y, Szép S, Lu Z (December 2009). "The antioxidant role of thiocyanate in the pathogenesis of cystic fibrosis and other inflammation-related diseases". Proceedings of the National Academy of Sciences of the United States of America 106 (48): 20515–19. Bibcode 2009PNAS..10620515X. doi:10.1073/pnas.0911412106. PMC 2777967. PMID 19918082. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2777967.
- ^ Moskwa P, Lorentzen D, Excoffon KJ, et al. (January 2007). "A novel host defense system of airways is defective in cystic fibrosis". American Journal of Respiratory and Critical Care Medicine 175 (2): 174–83. doi:10.1164/rccm.200607-1029OC. PMC 2720149. PMID 17082494. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2720149.
- ^ Conner GE, Wijkstrom-Frei C, Randell SH, Fernandez VE, Salathe M (January 2007). "The lactoperoxidase system links anion transport to host defense in cystic fibrosis". FEBS Letters 581 (2): 271–78. doi:10.1016/j.febslet.2006.12.025. PMC 1851694. PMID 17204267. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1851694.
- ^ a b Saiman L (2004). "Microbiology of early CF lung disease". Paediatric Respiratory Reviews 5 (Suppl A): S367–69. doi:10.1016/S1526-0542(04)90065-6. PMID 14980298.
- ^ Tümmler B, Koopmann U, Grothues D, Weissbrodt H, Steinkamp G, von der Hardt H (June 1991). "Nosocomial acquisition of Pseudomonas aeruginosa by cystic fibrosis patients". J. Clin. Microbiol. 29 (6): 1265–7. Bibcode 1991JPoSA..29.1265A. doi:10.1002/pola.1991.080290905. PMC 271975. PMID 1907611. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=271975.
- ^ Centers for Disease Control and Prevention (CDC) (June 1993). "Pseudomonas cepacia at summer camps for persons with cystic fibrosis". MMWR Morb. Mortal. Wkly. Rep. 42 (23): 456–9. PMID 7684813.
- ^ Pegues DA, Carson LA, Tablan OC, et al. (May 1994). "Acquisition of Pseudomonas cepacia at summer camps for patients with cystic fibrosis. Summer Camp Study Group". J. Pediatr. 124 (5 Pt 1): 694–702. doi:10.1016/S0022-3476(05)81357-5. PMID 7513755.
- ^ Pankhurst CL, Philpott-Howard J (April 1996). "The environmental risk factors associated with medical and dental equipment in the transmission of Burkholderia (Pseudomonas) cepacia in cystic fibrosis patients". J. Hosp. Infect. 32 (4): 249–55. doi:10.1016/S0195-6701(96)90035-3. PMID 8744509.
- ^ Jones AM, Govan JR, Doherty CJ, et al. (June 2003). "Identification of airborne dissemination of epidemic multiresistant strains of Pseudomonas aeruginosa at a CF centre during a cross infection outbreak". Thorax 58 (6): 525–27. doi:10.1136/thorax.58.6.525. PMC 1746694. PMID 12775867. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1746694.
- ^ Høiby N (June 1995). "Isolation and treatment of cystic fibrosis patients with lung infections caused by Pseudomonas (Burkholderia) cepacia and multiresistant Pseudomonas aeruginosa". Neth J Med 46 (6): 280–87. doi:10.1016/0300-2977(95)00020-N. PMID 7643943.
- ^ a b Pihet M, Carrere J, Cimon B, Chabasse D, Delhaes L, Symoens F, Bouchara JP (June 2009). "Occurrence and relevance of filamentous fungi in respiratory secretions of patients with cystic fibrosis--a review". Med Mycol. 47 (4): 387–97. doi:10.1080/13693780802609604. PMID 19107638.
- ^ PRapaka RR, Kolls JK (2009). "Pathogenesis of allergic bronchopulmonary aspergillosis in cystic fibrosis: current understanding and future directions". Med Mycol. 47 (Suppl. 1): S331–7. doi:10.1080/13693780802266777. PMID 18668399.
- ^ a b Davies JC, Alton EW, Bush A (December 2007). "Cystic fibrosis". BMJ 335 (7632): 1255–9. doi:10.1136/bmj.39391.713229.AD. PMC 2137053. PMID 18079549. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2137053.
- ^ Ross LF (September 2008). "Newborn screening for cystic fibrosis: a lesson in public health disparities". The Journal of Pediatrics 153 (3): 308–13. doi:10.1016/j.jpeds.2008.04.061. PMC 2569148. PMID 18718257. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2569148.
- ^ Assael, BM; Castellani C, Ocampo MB et al. (September 2002). "Epidemiology and survival analysis of cystic fibrosis in an area of intense neonatal screening over 30 years". American Journal of Epidemiology 156 (5): 397–401. doi:10.1093/aje/kwf064. PMID 12196308. http://aje.oxfordjournals.org/cgi/reprint/156/5/397.
- ^ Minarowski Ł, Sands D, Minarowska A, Karwowska A, Sulewska A, Gacko M, Chyczewska E. Thiocyanate concentration in saliva of cystic fibrosis patients. Folia Histochem Cytobiol. 2008;46(2):245-6. http://versita.metapress.com/content/12805r021413m867/fulltext.pdf
- ^ Stern RC (February 1997). "The diagnosis of cystic fibrosis". N. Engl. J. Med. 336 (7): 487–91. doi:10.1056/NEJM199702133360707. PMID 9017943.
- ^ Freudenheim, Milt (2009-12-22). "Tool in Cystic Fibrosis Fight: A Registry". New York Times: pp. D1. http://www.nytimes.com/2009/12/22/health/22cyst.html?8dpc=&pagewanted=all. Retrieved 2009-12-21.
- ^ American College of Obstetricians and Gynecologists and American College of Medical Genetics. Preconception and prenatal carrier screening for cystic fibrosis. Clinical and laboratory guidelines. American College of Obstetricians and Gynecologists, Washington, DC, October 2001.
- ^ Elias S, Annas GJ, Simpson JL (April 1991). "Carrier screening for cystic fibrosis: implications for obstetric and gynecologic practice". Am. J. Obstet. Gynecol. 164 (4): 1077–83. PMID 2014829.
- ^ Tabor A, Philip J, Madsen M, Bang J, Obel EB, Nørgaard-Pedersen B (June 1986). "Randomised controlled trial of genetic amniocentesis in 4606 low-risk women". Lancet 1 (8493): 1287–93. PMID 2423826.
- ^ Eddleman KA, Malone FD, Sullivan L, et al. (November 2006). "Pregnancy loss rates after midtrimester amniocentesis". Obstet Gynecol 108 (5): 1067–72. doi:10.1097/01.AOG.0000240135.13594.07. PMID 17077226.
- ^ Davis LB, Champion SJ, Fair SO, Baker VL, Garber AM (April 2010). "A cost-benefit analysis of preimplantation genetic diagnosis for carrier couples of cystic fibrosis". Fertil. Sterol. 93 (6): 1793–804. doi:10.1016/j.fertnstert.2008.12.053. PMID 19439290.
- ^ Pai VB, Nahata MC (October 2001). "Efficacy and safety of aerosolized tobramycin in cystic fibrosis". Pediatr. Pulmonol. 32 (4): 314–27. doi:10.1002/ppul.1125. PMID 11568993.
- ^ Westerman EM, Le Brun PP, Touw DJ, Frijlink HW, Heijerman HG (March 2004). "Effect of nebulized colistin sulphate and colistin sulphomethate on lung function in patients with cystic fibrosis: a pilot study". J. Cyst. Fibros. 3 (1): 23–8. doi:10.1016/j.jcf.2003.12.005. PMID 15463883.
- ^ McCoy KS, Quittner AL, Oermann CM, Gibson RL, Retsch-Bogart GZ, Montgomery AB (November 2008). "Inhaled aztreonam lysine for chronic airway Pseudomonas aeruginosa in cystic fibrosis". Am. J. Respir. Crit. Care Med. 178 (9): 921–8. doi:10.1164/rccm.200712-1804OC. PMC 2577727. PMID 18658109. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2577727.
- ^ Hansen CR, Pressler T, Koch C, Høiby N (March 2005). "Long-term azitromycin treatment of cystic fibrosis patients with chronic Pseudomonas aeruginosa infection; an observational cohort study". J. Cyst. Fibros. 4 (1): 35–40. doi:10.1016/j.jcf.2004.09.001. PMID 15752679.
- ^ Tan KH, Mulheran M, Knox AJ, Smyth AR (March 2003). "Aminoglycoside prescribing and surveillance in cystic fibrosis". Am. J. Respir. Crit. Care Med. 167 (6): 819–23. doi:10.1164/rccm.200109-012CC. PMID 12623858. http://ajrccm.atsjournals.org/cgi/pmidlookup?view=long&pmid=12623858.
- ^ van der Schans C, Prasad A, Main E (2000). Van Der Schans, Cees P. ed. "Chest physiotherapy compared to no chest physiotherapy for cystic fibrosis". Cochrane Database Syst Rev (2): CD001401. doi:10.1002/14651858.CD001401. PMID 10796781.
- ^ Kuver R, Lee SP (April 2006). "Hypertonic saline for cystic fibrosis". N. Engl. J. Med. 354 (17): 1848–51; author reply 1848–51. doi:10.1056/NEJMc060351. PMID 16642591.
- ^ Lieberman J (July 1968). "Dornase aerosol effect on sputum viscosity in cases of cystic fibrosis". JAMA 205 (5): 312–3. doi:10.1001/jama.205.5.312. PMID 5694947.
- ^ Kellerman, D.; Rossi Mospan, A.; Engels, J.; Schaberg, A.; Gorden, J.; Smiley, L. (2008). "Denufosol: A review of studies with inhaled P2Y2 agonists that led to Phase 3". Pulmonary Pharmacology & Therapeutics 21 (4): 600–607. doi:10.1016/j.pupt.2007.12.003. PMID 18276176.
- ^ Moran F, Bradley J (2003). Moran, Fidelma. ed. "Non-invasive ventilation for cystic fibrosis". Cochrane Database Syst Rev (2): CD002769. doi:10.1002/14651858.CD002769. PMID 12804435.
- ^ Huth MM, Zink KA, Van Horn NR (2005). "The effects of massage therapy in improving outcomes for youth with cystic fibrosis: an evidence review". Pediatr Nurs 31 (4): 328–32. PMID 16229132.
- ^ Fridell JA, Vianna R, Kwo PY, et al. (October 2005). "Simultaneous liver and pancreas transplantation in patients with cystic fibrosis". Transplant. Proc. 37 (8): 3567–9. doi:10.1016/j.transproceed.2005.09.091. PMID 16298663.
- ^ Belkin RA, Henig NR, Singer LG, et al. (March 2006). "Risk factors for death of patients with cystic fibrosis awaiting lung transplantation". Am. J. Respir. Crit. Care Med. 173 (6): 659–66. doi:10.1164/rccm.200410-1369OC. PMC 2662949. PMID 16387803. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2662949.
- ^ a b Alves CAD, Aguiar RA, Alves AC, Santana MA (April 2007). "Diabetes mellitus in patients with cystic fibrosis". J Bras Pneumol 33 (2): 213–21. doi:10.1590/S1806-37132007000200017. PMID 17724542.
- ^ Zirbes J, Milla CE (September 2009). "Cystic fibrosis related diabetes". Paediatr Respir Rev 10 (3): 118–23; quiz 123. doi:10.1016/j.prrv.2009.04.004. PMID 19651382.
- ^ Onady GM, Stolfi A (2005). Onady, Gary M. ed. "Insulin and oral agents for managing cystic fibrosis-related diabetes". Cochrane Database Syst Rev (3): CD004730. doi:10.1002/14651858.CD004730.pub2. PMID 16034943.
- ^ Conwell LS, Chang AB (2009). Conwell, Louise S. ed. "Bisphosphonates for osteoporosis in people with cystic fibrosis". Cochrane Database Syst Rev (4): CD002010. doi:10.1002/14651858.CD002010.pub2. PMID 19821288.
- ^ Hardin DS, Rice J, Ahn C, et al. (March 2005). "Growth hormone treatment enhances nutrition and growth in children with cystic fibrosis receiving enteral nutrition". J. Pediatr. 146 (3): 324–8. doi:10.1016/j.jpeds.2004.10.037. PMID 15756212.
- ^ Marks SC, Kissner DG (1997). "Management of sinusitis in adult cystic fibrosis". Am J Rhinol 11 (1): 11–4. doi:10.2500/105065897781446810. PMID 9065342.
- ^ Phillipson GT, Petrucco OM, Matthews CD (February 2000). "Congenital bilateral absence of the vas deferens, cystic fibrosis mutation analysis and intracytoplasmic sperm injection". Hum. Reprod. 15 (2): 431–5. doi:10.1093/humrep/15.2.431. PMID 10655317.
- ^ H. Yu,1 S. Z. Nasr,2 and V. Deretic, “Innate Lung Defenses and Compromised Pseudomonas aeruginosa Clearance in the Malnourished Mouse Model of Respiratory Infections in Cystic Fibrosis”, Infection and Immunity, http://iai.asm.org/cgi/content/abstract/68/4/2142 (accessed September 28, 2011)
- ^ Ratjen, F., & Do ̈ring, G. (2003). Cystic fibrosis. The Lancet, 361, 681–689. Rosenstein, B. J., & Zeitlin, P. L. (1998). Cystic fibrosis. Lancet, 351, 277–282.http://web.ebscohost.com/ehost/pdfviewer/pdfviewer?sid=f686ce3d-dbfc-4fcc-9aa6-dd772c42dc29%40sessionmgr13&vid=5&hid=17 (accessed September 28, 2011)
- ^ Schmitz, T. G. & Goldbeck, L. (2006) The effect of inpatient rehabilitation programmes on quality of life in patients with cystic fibrosis: a multi-centre study. Health Outcomes and Quality of Life, 4, 8–14. http://web.ebscohost.com/ehost/pdfviewer/pdfviewer?sid=bdb1ae75-163c-441d-a9d5-1c2db9643182%40sessionmgr14&vid=20&hid=11 (Accessed September 28, 2011)
- ^ Hegarty, M. M., MacDonald, J. J., Watter, P. P., & Wilson, C. C. (2009). Quality of life in young people with cystic fibrosis: effects of hospitalization, age and gender, and differences in parent/child perceptions. Child: Care, Health & Development, 35(4), 462-468. doi:10.1111/j.1365-2214.2008.00900.x ; Havermans, T., Vreys, M., Proesmans, M. & De Boeck, C. (2006) Assessment of agreement between parents and children on health-related quality of life in children with cystic fibrosis. Child: Care, Health and Development, 32, 1–7.http://web.ebscohost.com/ehost/pdfviewer/pdfviewer?sid=bdb1ae75-163c-441d-a9d5-1c2db9643182%40sessionmgr14&vid=20&hid=11 (Accessed September 28, 2011)
- ^ Moorcroft, A. J., Dodd, M. E., & Webb, A. K. (1998). Exercise limitations and training for patients with cystic fibrosis. Disability & Rehabilitation, 20(6/7), 247. Retrieved from EBSCOhost.
- ^ Cystic Fibrosis Canada. (2011). Medications. (Online catalogue No. 10684-5100 RR0001.). Retrieved from http://www.cysticfibrosis.ca/en/treatment/Medications.php
- ^ "What is the life expectancy for people who have CF (in the United States)?". Cystic Fibrosis Foundation. 2008. http://www.cff.org/aboutcf/faqs/#What_is_the_life_expectancy_for_people_who_have_CF_%28in_the_United_States%29?/. Retrieved 2010-03-14.
- ^ "Canadian Cystic Fibrosis Patient Data Registry Report" (PDF). Canadian Cystic Fibrosis Foundation. 2007. http://www.cysticfibrosis.ca/assets/files/pdf/CPDR_ReportE.pdf. Retrieved 2010-03-14.
- ^ "Cystic Fibrosis Patient Registry Annual Data Report 2008" (PDF). Cystic Fibrosis Foundation. 2008. http://www.cff.org/UploadedFiles/research/ClinicalResearch/2008-Patient-Registry-Report.pdf. Retrieved 2010-10-05.[dead link]
- ^ Araújo FG, Novaes FC, Santos NP, et al. (January 2005). "Prevalence of deltaF508, G551D, G542X, and R553X mutations among cystic fibrosis patients in the North of Brazil". Braz. J. Med. Biol. Res. 38 (1): 11–5. doi:10.1590/S0100-879X2005000100003. PMID 15665983.
- ^[unreliable medical source?]Ottawa university boots cystic fibrosis from charity drive, National Post, November 25, 2008
- ^ Cystic Fibrosis Foundation - Genetic Carrier Testing Updated 07/09/07
- ^ Rosenstein BJ, Cutting GR (April 1998). "The diagnosis of cystic fibrosis: a consensus statement. Cystic Fibrosis Foundation Consensus Panel". J. Pediatr. 132 (4): 589–95. doi:10.1016/S0022-3476(98)70344-0. PMID 9580754.
- ^ Hamosh A, FitzSimmons SC, Macek M, Knowles MR, Rosenstein BJ, Cutting GR (February 1998). "Comparison of the clinical manifestations of cystic fibrosis in black and white patients". J. Pediatr. 132 (2): 255–9. doi:10.1016/S0022-3476(98)70441-X. PMID 9506637.
- ^ Hytönen M, Patjas M, Vento SI, et al. (December 2001). "Cystic fibrosis gene mutations deltaF508 and 394delTT in patients with chronic sinusitis in Finland". Acta Otolaryngol. 121 (8): 945–7. PMID 11813900.
- ^[unreliable medical source?]About Cystic Fibrosis
- ^ Genetic testing for cystic fibrosis Genetic Testing for Cystic Fibrosis. National Institutes of Health, Consensus Development Conference Statement. April 14–16, 1997. Retrieved on November 20, 2009.
- ^ Rosenfeld M, Davis R, FitzSimmons S, Pepe M, Ramsey B (May 1997). "Gender gap in cystic fibrosis mortality". Am. J. Epidemiol. 145 (9): 794–803. PMID 9143209.
- ^ Coakley RD, Sun H, Clunes LA, et al. (December 2008). "17beta-Estradiol inhibits Ca2+-dependent homeostasis of airway surface liquid volume in human cystic fibrosis airway epithelia". J. Clin. Invest. 118 (12): 4025–35. doi:10.1172/JCI33893. PMC 2582929. PMID 19033671. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2582929.
- ^ Verma N, Bush A, Buchdahl R (October 2005). "Is there still a gender gap in cystic fibrosis?". Chest 128 (4): 2824–34. doi:10.1378/chest.128.4.2824. PMID 16236961.
- ^ Moran A, Dunitz J, Nathan B, Saeed A, Holme B, Thomas W (September 2009). "Cystic fibrosis-related diabetes: current trends in prevalence, incidence, and mortality". Diabetes Care 32 (9): 1626–31. doi:10.2337/dc09-0586. PMC 2732133. PMID 19542209. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2732133.
- ^ CF worse for women 'due to effect of oestregen' - The Irish Times - Tue, Aug 10, 2010
- ^ Wennberg C, Kucinskas V (1994). "Low frequency of the delta F508 mutation in Finno-Ugrian and Baltic populations". Hum. Hered. 44 (3): 169–71. doi:10.1159/000154210. PMID 8039801.
- ^ Kere J, Savilahti E, Norio R, Estivill X, de la Chapelle A (September 1990). "Cystic fibrosis mutation delta F508 in Finland: other mutations predominate". Hum. Genet. 85 (4): 413–5. doi:10.1007/BF02428286. PMID 2210753.
- ^ Wiuf C (August 2001). "Do delta F508 heterozygotes have a selective advantage?". Genet. Res. 78 (1): 41–7. PMID 11556136.
- ^ Gabriel SE, Brigman KN, Koller BH, Boucher RC, Stutts MJ (October 1994). "Cystic fibrosis heterozygote resistance to cholera toxin in the cystic fibrosis mouse model". Science 266 (5182): 107–9. Bibcode 1994Sci...266..107G. doi:10.1126/science.7524148. PMID 7524148.
- ^ Cuthbert AW, Halstead J, Ratcliff R, Colledge WH, Evans MJ (January 1995). "The genetic advantage hypothesis in cystic fibrosis heterozygotes: a murine study". J. Physiol. (Lond.) 482 ( Pt 2): 449–54. PMC 1157742. PMID 7714835. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1157742.
- ^ Högenauer C, Santa Ana CA, Porter JL, et al. (December 2000). "Active intestinal chloride secretion in human carriers of cystic fibrosis mutations: an evaluation of the hypothesis that heterozygotes have subnormal active intestinal chloride secretion". Am. J. Hum. Genet. 67 (6): 1422–7. doi:10.1086/316911. PMC 1287919. PMID 11055897. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1287919.
- ^ Pier GB, Grout M, Zaidi T, et al. (May 1998). "Salmonella typhi uses CFTR to enter intestinal epithelial cells". Nature 393 (6680): 79–82. Bibcode 1998Natur.393...79P. doi:10.1038/30006. PMID 9590693.
- ^ Modiano G, Ciminelli BM, Pignatti PF (March 2007). "Cystic fibrosis and lactase persistence: a possible correlation". Eur. J. Hum. Genet. 15 (3): 255–9. doi:10.1038/sj.ejhg.5201749. PMID 17180122.
- ^ Poolman EM, Galvani AP (February 2007). "Evaluating candidate agents of selective pressure for cystic fibrosis". Journal of the Royal Society, Interface 4 (12): 91–8. doi:10.1098/rsif.2006.0154. PMC 2358959. PMID 17015291. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2358959.
- ^ Williams, N (2006). "Footprint fears for new TB threat". Current Biology 16 (19): R821. doi:10.1016/j.cub.2006.09.009.
- ^ a b c Busch R (1990). "On the history of cystic fibrosis". Acta Univ Carol Med (Praha) 36 (1–4): 13–5. PMID 2130674.
- ^ Fanconi G., Uehlinger E., Knauer C. (1936). "Das coeliakiesyndrom bei angeborener zysticher pankreasfibromatose und bronchiektasien". Wien. Med. Wschr 86: 753–756.
- ^ Di Sant'Agnese PA, Darling RC, Perera GA, Shea E (November 1953). "Abnormal electrolyte composition of sweat in cystic fibrosis of the pancreas; clinical significance and relationship to the disease". Pediatrics 12 (5): 549–63. PMID 13111855.
- ^ Riordan JR, Rommens JM, Kerem B, et al. (September 1989). "Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA". Science 245 (4922): 1066–73. Bibcode 1989Sci...245.1066R. doi:10.1126/science.2475911. PMID 2475911.
- ^ Rommens JM, Iannuzzi MC, Kerem B, et al. (September 1989). "Identification of the cystic fibrosis gene: chromosome walking and jumping". Science 245 (4922): 1059–65. Bibcode 1989Sci...245.1059R. doi:10.1126/science.2772657. PMID 2772657.
- ^ Ramalho AS, Beck S, Meyer M, Penque D, Cutting GR, Amaral MD (November 2002). "Five percent of normal cystic fibrosis transmembrane conductance regulator mRNA ameliorates the severity of pulmonary disease in cystic fibrosis". Am. J. Respir. Cell Mol. Biol. 27 (5): 619–27. PMID 12397022.
- ^ Tate S, Elborn S (March 2005). "Progress towards gene therapy for cystic fibrosis". Expert Opin Drug Deliv 2 (2): 269–80. doi:10.1517/17425247.2.2.269. PMID 16296753.
- ^ redirect
- ^ Dietz, HC; Guttmacher, Alan E.; Dietz, Harry C. (August 2010). "New therapeutic approaches to Mendelian disorders". N. Engl. J. Med. 363 (9): 852863. doi:10.1056/NEJMra0907180. PMID 20818846. http://www.nejm.org/doi/full/10.1056/NEJMra0907180. Free full text
- ^ Wilschanski M, Yahav Y, Yaacov Y, et al. (October 2003). "Gentamicin-induced correction of CFTR function in patients with cystic fibrosis and CFTR stop mutations". N. Engl. J. Med. 349 (15): 1433–41. doi:10.1056/NEJMoa022170. PMID 14534336. http://content.nejm.org/cgi/content/full/349/15/1433.
- ^ ClinicalTrials.gov NCT00803205 Study of Ataluren (PTC124™) in Cystic Fibrosis
- ^ Merk; Schubert-Zsilavecz. (in German)Pharmazeutische Zeitung 156 (37): 24–27.
Further reading
- Fungal etiology Um i think in CF-associated infections reviewed extensively by Pihet et al.: Pihet M, Carrere J, Cimon B, Chabasse D, Delhaes L, Symoens F, Bouchara JP (June 2009). "Occurrence and relevance of filamentous fungi in respiratory secretions of patients with cystic fibrosis--a review". Med Mycol. 47 (4): 387–97. doi:10.1080/13693780802609604. PMID 19107638.
- Mowska, Patryk, Daniel Lorentzen, Katherine Excoffon, Joseph Zabner, Paul B. McCray, William M. Nauseef, Corinne Dupuy, and Botond Bánfi. A novel host defense system of airways is defective in cystic fibrosis. American Journal of Respiratory and Critical Care Medicine, 1 Nov. 2006. Web. 26 Nov. 2009
- Childers M, Eckel G, Himmel A, Caldwell J. A new model of cystic fibrosis pathology: lack of transport of glutathion and its thiocyanate conjugates. Med Hypotheses. 2007;68(1):101-12.
- Conner GE, Salathe M, Forteza R Lactoperoxidase and hydrogen peroxide metabolism in the airway, AmJ Respir Crit Care Med 2002 Dec 15;166 (12 Pt2):S57-1 Review
- Conner GE, Wijkstrom-Frei C, Randell SH, Fernandez VE, Salathe M. The lactoperoxidase system links anion transport to host defense in cystic fibrosis. FEBS Lett. 2007;581(2):271-8.
- Minarowski Ł, Sands D, Minarowska A, Karwowska A, Sulewska A, Gacko M, Chyczewska E. Thiocyanate concentration in saliva of cystic fibrosis patients. Folia Histochem Cytobiol. 2008;46(2):245-6.
- Rada B, Leto TL. Redox warfare between airway epithelial cells and Pseudomonas : dual oxidase versus pyocyanin. Immunol. Res. 2008
- Fischer H. Mechanism and function of DUOX in epithelia of the lung. Antioxid Redox Signal. 2009;11(10):1-13.
- Pedemonte N, Caci E, Sondo E, Caputo A, et al. Thiocyanate transport in resting & IL-4-stimulated human bronchial epithelial cells: role of pendrin and anion channels. J Immunol. 2007;178(8):5144-53.
- Wijkstrom-Frei C, El-Chemaly S, Ali-Rachedi R, Gerson C, Cobas MA, Forteza R, Salathe M, Conner GE. Lactoperoxidase and human airway host defense. Am J Respir Cell Mol Biol 2003;29(2):206-12.
- Xu Y, Szep S, Lu Z. The antioxidant role of thiocyanate in the pathogenesis of cystic fibrosis and other inflammation related diseases, PNAS. 2009; Early edition, November 16
External links
- Cystic fibrosis at the Open Directory Project
- Cystic fibrosis pictures (Univ Geneva, Switzerland)
- cf at NIH/UW GeneTests
- Rare Diseases Clinical Research Network
- Search GeneCards for genes involved in Cystic Fibrosis
- Cystic Fibrosis Mutation Database
- Online Mendelian Inheritance of Man summary of Cystic Fibrosis
Cardiopulmonary therapy Diagnostic Pulmonary function testing • PolysomnographyDisease Therapy Hyperinflation therapy • Pulmonary hygiene • Mechanical ventilation • Oxygen therapySee also Genetic disorder, membrane: ABC-transporter disorders ABCA ABCA1 (Tangier disease) · ABCA3 (Surfactant metabolism dysfunction 3) · ABCA4 (Stargardt disease 1, Retinitis pigmentosa 19) · ABCA12 (Harlequin-type ichthyosis, Lamellar ichthyosis 2)ABCB ABCC ABCC2 (Dubin–Johnson syndrome) · ABCC6 (Pseudoxanthoma elasticum) · ABCC7 (Cystic fibrosis) · ABCC8 (HHF1, TNDM2) · ABCC9 (Dilated cardiomyopathy 1O)ABCD ABCG see also ABC transporters
B structural (perx, skel, cili, mito, nucl, sclr) · DNA/RNA/protein synthesis (drep, trfc, tscr, tltn) · membrane (icha, slcr, atpa, abct, othr) · transduction (iter, csrc, itra), trfkCategories:- Channelopathy
- Autosomal recessive disorders
- Pancreas disorders
- Pediatrics
- Lung disorders
- Congenital disorders
- Rare diseases
Wikimedia Foundation. 2010.