- Flow chemistry
-
In flow chemistry, a chemical reaction is run in a continuously flowing stream rather than in batch production. In other words, pumps move fluid into a tube, and where tubes join one another, the fluids contact one another. If these fluids are reactive, a reaction takes place. Flow chemistry is a well-established technique for use at a large scale when manufacturing large quantities of a given material. However, it is relatively new to use it in the laboratory environment.[1]
Contents
Batch vs. flow
Comparing parameters in Batch vs Flow:
- Reaction stoichiometry. In batch production this is defined by the concentration of chemical reagents and their volumetric ratio. In Flow this is defined by the concentration of reagents and the ratio of their flow rate.
- Residence time. In batch production this is determined by how long a vessel is held at a given temperature. In flow this is determined by the volume of the reactor, and the bulk flow rate.
Benefits of flow
- Reaction temperature can be far above the solvent's boiling point due to easy ability to contain pressure.
- Mixing can be achieved within seconds at the smaller scales used in flow chemistry.
- The thermal mass of the fluid is typically far lower than the thermal mass of the system (and orders of magnitude less than with batch chemistry). This makes controlling the temperature of the media both faster and easier ensuring that exothermic and endothermic process can be conducted without issue.
- Multi step reactions can be arranged in a continuous sequence. This can be especially beneficial if intermediate compounds are unstable, since they will exist only momentarily and in very small quantities.
- Position along the flowing stream and reaction time point are directly related to one another. This means that it is possible to arrange the system such that further reagents can be introduced into the flowing reaction stream at precisely the time point in the reaction that is desired.
- It is possible to arrange a flowing system such that purification is coupled with the reaction. There are three primary techniques that are used:
- Solid phase scavenging
- Chromatographic separation
- Liquid/Liquid Extraction
- By coupling the output of the reactor to a detector system, it is possible with appropriate controls to create an unattended system which can sequentially investigate a range of possible reaction parameters (varying stoichiometry, residence time and temperature) and therefore optimise reactions with little or no intervention.
- Reactions which involve reagents containing dissolved gases are easily handled, whereas in batch a pressurised "bomb" reactor would be necessary.
- Multi phase liquid reactions (e.g. phase transfer catalysis) can be performed in a straightforward way, with high reproducibility over a range of scales and conditions.
- Scaleup of a proven reaction can be achieved rapidly with little or no process development work, by either changing the reactor volume or by running several reactors in parallel, provided that flows are recalculated to achieve the same residence times.
Continuous flow reactor
Continuous reactors are typically tube like and manufactured from non-reactive materials such as stainless steel, glass and polymers. Mixing methods include diffusion alone (if the diameter of the reactor is small e.g. <1 mm) and static mixers.
Continuous flow reactors allow good control over reaction conditions including heat transfer, time and mixing.
The residence time of the reagents in the reactor (i.e. the amount of time that the reaction is heated or cooled) is calculated from the volume of the reactor and the flow rate through it.
Residence time = Reactor Volume / Flow Rate
Therefore, to achieve a longer residence time, reagents can be pumped more slowly and/or a larger volume reactor used. Production rates can vary from nano litres to litres per minute.
Some examples of flow reactors are spinning disc reactors (Colin Ramshaw); spinning tube reactors; multi-cell flow reactors; oscillatory flow reactors; microreactors; hex reactors; and 'aspirator reactors'.
In an aspirator reactor a pump propels one reagent, which causes a reactant to be sucked in. This type of reactor was patented circa 1941 by Nobel companies for used in preparing nitroglycerin.
Flow reactor scale
The smaller scale of micro flow reactors can make them ideal for process development experiments. Although it is possible to operate flow processes at a Tonne plus scale, synthetic efficiency benefits from improved thermal and mass transfer as well as mass transport.
File:X-cube.jpga microreactorUse of gases in flow
Laboratory scale flow reactors are ideal systems for using gases, particularly those that are toxic or associated with other hazards. The gas reactions that have been most successfully adapted to flow are Hydrogenation and Carbonylation, although work has also been performed using other gases, from ethylene to ozone.
Reasons for the suitability of flow systems for hazardous gas handling are:
- Systems allow the use of a fixed bed catalyst. Combined with low solution concentrations, this allows all compound to be adsorbed to catalyst in the presence of gas
- Comparatively small amounts of gas are continually exhausted by the system, eliminating the need for many of the special precautions normally required for handling toxic and/or flammable gases
- The addition of pressure means that a far greater proportion of the gas will be in solution during the reaction than is the case conventionally
- The greatly enhanced mixing of the solid, liquid and gaseous phases allows the researcher to exploit the kinetic benefits of elevated temperatures without being concerned about the gas being displaced from solution
Other uses of flow
It is possible to run experiments in flow using more sophisticated techniques, such as solid phase chemistries.
Scale up of microwave reactions
Microwave reactors are frequently used for small scale batch chemistry. However due to the extremes of temperature and pressure reached in a microwave it is often difficult to transfer these reactions to conventional non-microwave apparatus for subsequent development, leading to difficulties with scaling studies. A flow reactor with suitable high temperature ability and pressure control can directly and accurately mimic the conditions created in a microwave reactor, and since in flow chemistry the quantity of material produced is limited only by the reaction time, scaling can be achieved.
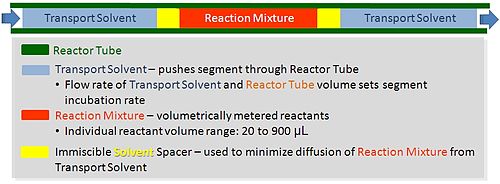
Segmented Flow Chemistry
As discussed above, running experiments in continuous flow systems is difficult, especially when one is developing new chemical reactions, which requires screening of multiple components, varying stoichiometry, temperature and incubation time (flow rate).
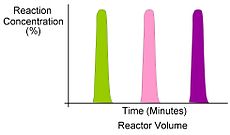
In continuous flow, experiments are performed serially, which means only one experimental condition can populate the reactor tube at any given time. Experimental throughput is highly variable and is dependent upon the reactor volume and residence time (flow rate).
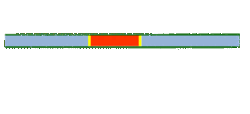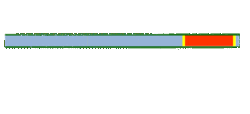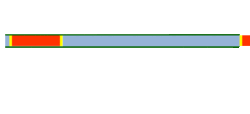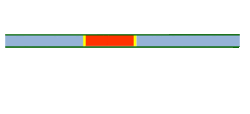
Segmented flow is an approach that improves upon the speed in which screening, optimization and libraries can be conducted in flow chemistry. Segmented flow uses a "Plug Flow" approach where specific volumetric experimental mixtures are created and then injected into a high pressure flow reactor. Diffusion of the segment (reaction mixture) is minimized by using immiscible solvent on the leading and rear ends of the segment.
One of the primary benefits of segmented flow chemistry is the ability to run experiments in a serial/parallel manner where experiments that share the same residence time and temperature can be repeatedly created and injected. In addition, the volume of each experiment is independent to that of the volume of the flow tube thereby saving a significant amount of reactant per experiment. When performing reaction screening and libraries, segment composition is typically varied by composition of matter. When performing reaction optimization, segments vary by stoichiometry.
Segmented flow is also used with online LCMS, both analytical and preparative where the segments are detected when exiting the reactor using UV and subsequently diluted for analytical LCMS or injected directly for preparative LCMS.
See also
References
- ^ A. Kirschning (Editor): Chemistry in flow systems and Chemistry in flow systems II Thematic Series in the Open Access Beilstein Journal of Organic Chemistry.
External links
Continuous flow multi-step organic synthesis - a Chemical Science Mini Review by Damien Webb and Timothy F. Jamison discussing the current state of the art and highlighting recent progress and current challenges facing the emerging area of continuous flow techniques for multi-step synthesis. Published by the Royal Society of Chemistry
Categories:
Wikimedia Foundation. 2010.