- Colloid
-
A colloid is a substance microscopically dispersed evenly throughout another substance.[1]
A colloidal system consists of two separate phases: a dispersed phase (or internal phase) and a continuous phase (or dispersion medium). A colloidal system may be solid, liquid, or gaseous.
Many familiar substances are colloids, as shown in the chart below. In addition to these naturally occurring colloids, modern chemical process industries utilize high shear mixing technology to create novel colloids.
The dispersed-phase particles have a diameter of between approximately 5 and 200 nanometers.[2] Such particles are normally invisible in an optical microscope, though their presence can be confirmed with the use of an ultramicroscope or an electron microscope. Homogeneous mixtures with a dispersed phase in this size range may be called colloidal aerosols, colloidal emulsions, colloidal foams, colloidal dispersions, or hydrosols. The dispersed-phase particles or droplets are affected largely by the surface chemistry present in the colloid.
Some colloids are translucent because of the Tyndall effect, which is the scattering of light by particles in the colloid. Other colloids may be opaque or have a slight color.
Colloidal solutions (also called colloidal suspensions) are the subject of interface and colloid science. This field of study was introduced in 1861 by Scottish scientist Thomas Graham.
Classification
Because the size of the dispersed phase may be difficult to measure, and because colloids have the appearance of solutions, colloids are sometimes identified and characterized by their physico-chemical and transport properties. For example, if a colloid consists of a solid phase dispersed in a liquid, the solid particles will not diffuse through a membrane, whereas with a true solution the dissolved ions or molecules will diffuse through a membrane. Because of the size exclusion, the colloidal particles are unable to pass through the pores of an ultrafiltration membrane with a size smaller than their own dimension. The smaller the size of the pore of the ultrafiltration membrane, the lower the concentration of the dispersed colloidal particules remaining in the ultrafiltred liquid. The exact value of the concentration of a truly dissolved species will thus depend on the experimental conditions applied to separate it from the colloidal particles also dispersed in the liquid. This is, a.o., particularly important for solubility studies of readily hydrolysed species such as Al, Eu, Am, Cm, ... or organic matter complexing these species. Colloids can be classified as follows:
Medium / Phases Dispersed phase Gas Liquid Solid Continuous medium Gas NONE
(All gases are mutually miscible)Liquid aerosol
Examples: fog, mist, hair spraysSolid aerosol
Examples: smoke, cloud, air particulatesLiquid Foam
Example: whipped cream, Shaving creamEmulsion
Examples: milk, mayonnaise, hand creamSol
Examples: pigmented ink, bloodSolid Solid foam
Examples: aerogel, styrofoam, pumiceGel
Examples: agar, gelatin, jelly, opalSolid sol
Example: cranberry glassIn some cases, a colloid can be considered as a homogeneous mixture. This is because the distinction between "dissolved" and "particulate" matter can be sometimes a matter of approach, which affects whether or not it is homogeneous or heterogeneous.
Hydrocolloids
A hydrocolloid is defined as a colloid system wherein the colloid particles are dispersed in water. A hydrocolloid has colloid particles spread throughout water, and depending on the quantity of water available that can take place in different states, e.g., gel or sol (liquid). Hydrocolloids can be either irreversible (single-state) or reversible. For example, agar, a reversible hydrocolloid of seaweed extract, can exist in a gel and sol state, and alternate between states with the addition or elimination of heat.
Many hydrocolloids are derived from natural sources. For example, agar-agar and carrageenan are extracted from seaweed, gelatin is produced by hydrolysis of proteins of bovine and fish origins, and pectin is extracted from citrus peel and apple pomace.
Gelatin desserts like jelly or Jell-O are made from gelatin powder, another effective hydrocolloid. Hydrocolloids are employed in food mainly to influence texture or viscosity (e.g., a sauce). Hydrocolloid-based medical dressings are used for skin and wound treatment.
Other main hydrocolloids are xanthan gum, gum arabic, guar gum, locust bean gum, cellulose derivatives as carboxymethyl cellulose, alginate and starch.
Interaction between colloid particles
The following forces play an important role in the interaction of colloid particles:
- Excluded volume repulsion: This refers to the impossibility of any overlap between hard particles.
- Electrostatic interaction: Colloidal particles often carry an electrical charge and therefore attract or repel each other. The charge of both the continuous and the dispersed phase, as well as the mobility of the phases are factors affecting this interaction.
- van der Waals forces: This is due to interaction between two dipoles that are either permanent or induced. Even if the particles do not have a permanent dipole, fluctuations of the electron density gives rise to a temporary dipole in a particle. This temporary dipole induces a dipole in particles nearby. The temporary dipole and the induced dipoles are then attracted to each other. This is known as van der Waals force, and is always present (unless the refractive indexes of the dispersed and continuous phases are matched), is short-range, and is attractive.
- Entropic forces: According to the second law of thermodynamics, a system progresses to a state in which entropy is maximized. This can result in effective forces even between hard spheres.
- Steric forces between polymer-covered surfaces or in solutions containing non-adsorbing polymer can modulate interparticle forces, producing an additional steric repulsive force (which is predominantly entropic in origin) or an attractive depletion force between them. Such an effect is specifically searched for with tailor-made superplasticizers developed to increase the workability of concrete and to reduce its water content.
Preparation of colloids
There are two principal ways of preparation of colloids:[3]
- Dispersion of large particles to the colloidal dimensions;
- Condensation of molecules dissolved in a true solution into larger colloidal particles.
Stabilization of a colloidal dispersion (peptization)
Stabilization serves to prevent colloids from aggregating. Steric stabilization and electrostatic stabilization are the two main mechanisms for colloid stabilization. Electrostatic stabilization is based on the mutual repulsion of like electrical charges. In general, different phases have different charge affinities, so that a electrical double layer forms at any interface. Small particle sizes lead to enormous surface areas, and this effect is greatly amplified in colloids. In a stable colloid, mass of a dispersed phase is so low that its buoyancy or kinetic energy is too weak to overcome the electrostatic repulsion between charged layers of the dispersing phase. The charge on the dispersed particles can be observed by applying an electric field: All particles migrate to the same electrode and therefore must all have the same sign charge.
Destabilizing a colloidal dispersion (flocculation)
Unstable colloidal dispersions form flocs as the particles aggregate due to interparticle attractions. In this way photonic glasses can be grown. This can be accomplished by a number of different methods:
- Removal of the electrostatic barrier that prevents aggregation of the particles. This can be accomplished by the addition of salt to a suspension or changing the pH of a suspension to effectively neutralize or "screen" the surface charge of the particles in suspension. This removes the repulsive forces that keep colloidal particles separate and allows for coagulation due to van der Waals forces.
- Addition of a charged polymer flocculant. Polymer flocculants can bridge individual colloidal particles by attractive electrostatic interactions. For example, negatively-charged colloidal silica or clay particles can be flocculated by the addition of a positively-charged polymer.
- Addition of non-adsorbed polymers called depletants that cause aggregation due to entropic effects.
- Physical deformation of the particle (e.g., stretching) may increase the van der Waals forces more than stabilization forces (such as electrostatic), resulting coagulation of colloids at certain orientations.
Unstable colloidal suspensions of low-volume fraction form clustered liquid suspensions, wherein individual clusters of particles fall to the bottom of the suspension (or float to the top if the particles are less dense than the suspending medium) once the clusters are of sufficient size for the Brownian forces that work to keep the particles in suspension to be overcome by gravitational forces. However, colloidal suspensions of higher-volume fraction form colloidal gels with viscoelastic properties. Viscoelastic colloidal gels, such as bentonite and toothpaste, flow like liquids under shear, but maintain their shape when shear is removed. It is for this reason that toothpaste can be squeezed from a toothpaste tube, but stays on the toothbrush after it is applied.
Technique monitoring colloidal stability
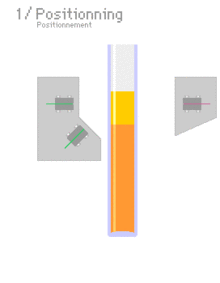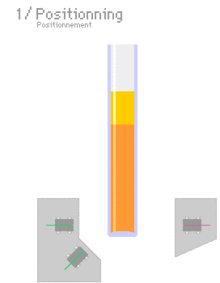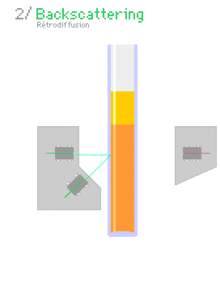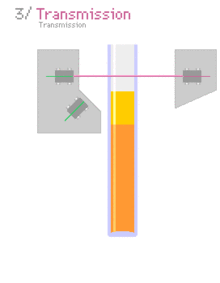
Multiple light scattering coupled with vertical scanning is the most widely used technique to monitor the dispersion state of a product, hence identifying and quantifying destabilisation phenomena.[4][5][6][7] It works on concentrated dispersions without dilution. When light is sent through the sample, it is backscattered by the particles / droplets. The backscattering intensity is directly proportional to the size and volume fraction of the dispersed phase. Therefore, local changes in concentration (e.g.Creaming and Sedimentation) and global changes in size (e.g. flocculation, coalescence) are detected and monitored.
Accelerating methods for shelf life prediction
The kinetic process of destabilisation can be rather long (up to several months or even years for some products) and it is often required for the formulator to use further accelerating methods in order to reach reasonable development time for new product design. Thermal methods are the most commonly used and consists in increasing temperature to accelerate destabilisation (below critical temperatures of phase inversion or chemical degradation). Temperature affects not only the viscosity, but also interfacial tension in the case of non-ionic surfactants or more generally interactions forces inside the system. Storing a dispersion at high temperatures enables to simulate real life conditions for a product (e.g. tube of sunscreen cream in a car in the summer), but also to accelerate destabilisation processes up to 200 times. Mechanical acceleration including vibration, centrifugation and agitation are sometimes used. They subject the product to different forces that pushes the particles / droplets against one another, hence helping in the film drainage. However, some emulsions would never coalesce in normal gravity, while they do under artificial gravity.[8] Moreover segregation of different populations of particles have been highlighted when using centrifugation and vibration.[9]
Colloids as a model system for atoms
In physics, colloids are an interesting model system for atoms. Micrometre-scale colloidal particles are large enough to be observed by optical techniques such as confocal microscopy. Many of the forces that govern the structure and behavior of matter, such as excluded volume interactions or electrostatic forces, govern the structure and behavior of colloidal suspensions. For example, the same techniques used to model ideal gases can be applied to model the behavior of a hard sphere colloidal suspension. In addition, phase transitions in colloidal suspensions can be studied in real time using optical techniques, and are analogous to phase transitions in liquids.
Colloidal crystals
Main article: Colloidal crystalA colloidal crystal is a highly ordered array of particles that can be formed over a very long range (typically on the order of a few millimeters to one centimeter) and that appear analogous to their atomic or molecular counterparts.[10] One of the finest natural examples of this ordering phenomenon can be found in precious opal, in which brilliant regions of pure spectral color result from close-packed domains of amorphous colloidal spheres of silicon dioxide (or silica, SiO2).[11][12] These spherical particles precipitate in highly siliceous pools in Australia and elsewhere, and form these highly ordered arrays after years of sedimentation and compression under hydrostatic and gravitational forces. The periodic arrays of submicrometre spherical particles provide similar arrays of interstitial voids, which act as a natural diffraction grating for visible light waves, particularly when the interstitial spacing is of the same order of magnitude as the incident lightwave.[13][14]
Thus, it has been known for many years that, due to repulsive Coulombic interactions, electrically charged macromolecules in an aqueous environment can exhibit long-range crystal-like correlations with interparticle separation distances, often being considerably greater than the individual particle diameter. In all of these cases in nature, the same brilliant iridescence (or play of colors) can be attributed to the diffraction and constructive interference of visible lightwaves that satisfy Bragg’s law, in a matter analogous to the scattering of X-rays in crystalline solids.
The large number of experiments exploring the physics and chemistry of these so-called "colloidal crystals" has emerged as a result of the relatively simple methods that have evolved in the last 20 years for preparing synthetic monodisperse colloids (both polymer and mineral) and, through various mechanisms, implementing and preserving their long-range order formation.
Colloids in biology
In the early 20th century, before enzymology was well understood, colloids were thought to be the key to the operation of enzymes; i.e., the addition of small quantities of an enzyme to a quantity of water would, in some fashion yet to be specified, subtly alter the properties of the water so that it would break down the enzyme's specific substrate,[citation needed] such as a solution of ATPase breaking down ATP. Furthermore, life itself was explainable in terms of the aggregate properties of all the colloidal substances that make up an organism. As more detailed knowledge of biology and biochemistry developed, the colloidal theory was replaced by the macromolecular theory, which explains an enzyme as a collection of identical huge molecules that act as very tiny machines, freely moving about between the water molecules of the solution and individually operating on the substrate, no more mysterious than a factory full of machinery. The properties of the water in the solution are not altered, other than the simple osmotic changes that would be caused by the presence of any solute. In humans, both the thyroid gland and the intermediate lobe (pars intermedia) of the pituitary gland contain colloid follicles.
Colloids in the environment
Colloidal particles can also serve as transport vector[15] of diverse contaminants in the surface water (sea water, lakes, rivers, fresh water bodies) and in underground water circulating in fissured rocks[16] (limestone, sandstone, granite, ...). Radionuclides and heavy metals easily sorb onto colloids suspended in water. Various types of colloids are recognised: inorganic colloids (clay particles, silicates, iron oxy-hydroxides, ...), organic colloids (humic and fulvic substances). When heavy metals or radionuclides form their own pure colloids, the term "Eigencolloid" is used to designate pure phases, e.g., Tc(OH)4, U(OH)4, Am(OH)3. Colloids have been suspected for the long-range transport of plutonium on the Nevada Nuclear Test Site. They have been the subject of detailed studies for many years. However, the mobility of inorganic colloids is very low in compacted bentonites and in deep clay formations[17] because of the process of ultrafiltration occurring in dense clay membrane.[18] The question is less clear for small organic colloids often mixed in porewater with truly dissolved organic molecules.[19]
Use in intravenous therapy
Colloid solutions used in intravenous therapy belong to a major group of volume expanders, and can be used for intravenous fluid replacement. Colloids preserve a high colloid osmotic pressure in the blood,[20] and therefore, they should theoretically preferentially increase the intravascular volume, whereas other types of volume expanders called crystalloids also increases the interstitial volume and intracellular volume. However, there is still controversy to the actual difference in efficacy by this difference.[20] Another difference is that crystalloids generally are much cheaper than colloids.[20] Recently, however, it has been determined that the use of colloids was bolstered by faked research studies.[21]
See also
- Aerosol
- Bacteriophage
- Colloid-facilitated transport
- Dispersion
- Eigencolloid
- Electrical double layer (EDL)
- Emulsion
- Entropic force
- Flocculation
- Foam
- Gel
- Gum (botany)
- Hydrosol
- Interface
- Miscibility
- Micromeritics
- Nanoparticle
- Non-Newtonian fluid
- Peptization
- Sol (colloid)
- Sol-gel
- Streaming potential
- Superplasticizer
- Suspension
- Zeta potential
References
- ^ "Colloid". Britannica Online Encyclopedia. http://www.britannica.com/EBchecked/topic/125898/colloid. Retrieved 2009-08-31.
- ^ Levine, Ira N. (2001). Physical Chemistry (5th ed.). Boston: McGraw-Hill. ISBN 0-07-231808-2., p. 955
- ^ http://www.substech.com/dokuwiki/doku.php?id=preparation_of_colloids
- ^ Roland, I; Piel, G; Delattre, L; Evrard, B (2003). "Systematic characterization of oil-in-water emulsions for formulation design". International Journal of Pharmaceutics 263 (1–2): 85–94. doi:10.1016/S0378-5173(03)00364-8. PMID 12954183.
- ^ Lemarchand, Caroline; Couvreur, Patrick; Besnard, Madeleine; Costantini, Dominique; Gref, Ruxandra (2003). "Novel polyester-polysaccharide nanoparticles". Pharmaceutical Research 20 (8): 1284–92. doi:10.1023/A:1025017502379. PMID 12948027.
- ^ Mengual, O (1999). "Characterisation of instability of concentrated dispersions by a new optical analyser: the TURBISCAN MA 1000". Colloids and Surfaces A: Physicochemical and Engineering Aspects 152: 111. doi:10.1016/S0927-7757(98)00680-3.
- ^ P. Bru et al. (2004). T. Provder and J. Texter. ed. Particle sizing and characterisation.
- ^ J-L Salager (2000). Françoise Nielloud,Gilberte Marti-Mestres. ed. Pharmaceutical emulsions and suspensions. CRC press. p. 89. ISBN 0824703049. http://books.google.com/?id=hDOS5OfL_pQC&pg=PA89&lpg=PA89.
- ^ Snabre, Patrick; Pouligny, Bernard (2008). "Size Segregation in a Fluid-like or Gel-like Suspension Settling under Gravity or in a Centrifuge". Langmuir 24 (23): 13338–47. doi:10.1021/la802459u. PMID 18986182.
- ^ Pieranski, P. (1983). "Colloidal Crystals". Contemporary Physics 24: 25. Bibcode 1983ConPh..24...25P. doi:10.1080/00107518308227471.
- ^ Sanders, J.V.; Sanders, J. V.; Segnit, E. R. (1964). "Structure of Opal". Nature 204 (4962): 1151. Bibcode 1964Natur.204..990J. doi:10.1038/204990a0.
- ^ Darragh, P.J., et al. (1976). Scientific American 234: 84.
- ^ Luck, W. et al. (1963). "Ber. Busenges". Phys. Chem. 67: 84.
- ^ Hiltner, P.A. and Krieger, I.M. (1969). "Diffraction of light by ordered suspensions". J. Phys. Chem. 73 (7): 2306. doi:10.1021/j100727a049.
- ^ Frimmel, Fritz H.; Frank von der Kammer, Hans-Curt Flemming (2007). Colloidal transport in porous media (1 ed.). Springer. p. 292. ISBN 3540713387. http://www.springer.com/earth+sciences/book/978-3-540-71338-8?detailsPage=toc.
- ^ Alonso, U.; T. Missana, A. Patelli, V. Rigato (2007). "Bentonite colloid diffusion through the host rock of a deep geological repository". Physics and Chemistry of the Earth, Parts A/B/C 32 (1–7): 469–476. Bibcode 2007PCE....32..469A. doi:10.1016/j.pce.2006.04.021. ISSN 1474-7065.
- ^ Voegelin, A.; Kretzschmar, R. (December 2002). Stability and mobility of colloids in Opalinus Clay.. Nagra Technical Report 02-14.. Institute of Terrestrial Ecology, ETH Zürich. p. 47. ISSN 1015-2636. http://www.nagra.ch/documents/database/dokumente/%24default/Default%20Folder/Publikationen/e%5Fntb02%2D14.pdf.
- ^ "Diffusion of colloids in compacted bentonite". http://www.kth.se/che/divisions/nuchem/research/1.19965?l=en_UK. Retrieved 2009-02-12.
- ^ Wold, Susanna; Trygve Eriksen (2007). "Diffusion of humic colloids in compacted bentonite". Physics and Chemistry of the Earth, Parts A/B/C 32 (1–7): 477–484. Bibcode 2007PCE....32..477W. doi:10.1016/j.pce.2006.05.002. ISSN 1474-7065.
- ^ a b c An Update on Intravenous Fluids by Gregory S. Martin, MD, MSc
- ^ Millions of surgery patients at risk in drug research fraud scandal, The Telegraph, 04 November 2011
Further reading
- Lyklema, J. Fundamentals of Interface and Colloid Science, Vol. 2, p. 3208, 1995
- Hunter, R.J. Foundations of Colloid Science, Oxford University Press, 1989
- Dukhin, S.S. & Derjaguin, B.V. Electrokinetic Phenomena, J.Wiley and Sons, 1974
- Russel, W.B., Saville, D.A. and Schowalter, W.R. Colloidal Dispersions, Cambridge, 1989 Cambridge University Press
- Kruyt, H.R. Colloid Science, Volume 1, Irreversible systems, Elsevier, 1959
- Dukhin, A.S. and Goetz, P.J. Ultrasound for characterizing colloids, Elsevier, 2002
- Rodil, Ma. Lourdes C., Chemistry The Central Science, 7th Ed. ISBN 013533480
- Pieranski, P., Colloidal Crystals, Contemp. Phys., Vol. 24, p. 25 (1983)
- Sanders, J.V., Structure of Opal, Nature, Vol. 204, p. 1151, (1964);
- Darragh, P.J., et al., Scientific American, Vol. 234, p. 84, (1976)
- Luck, W. et al., Ber. Busenges Phys. Chem., Vol. 67, p. 84 (1963);
- Hiltner, P.A. and Krieger, I.M., Diffraction of Light by Ordered Suspensions, J. Phys. Chem., Vol. 73, p. 2306 (1969)
- Arora, A.K., Tata, B.V.R., Eds. Ordering & Phase Transitions in Charged Colloids Wiley, New York (1996)
- Sood, A.K. in Solid State Physics, Eds. Ehrenreich, H., Turnbull, D., Vol. 45, p. 1 (1991)
- Murray, C.A. and Grier, D.G., Colloidal Crystals, Amer. Scientist, Vol. 83, p. 238 (1995);
- Video Microscopy of Monodisperse Colloidal Systems, Ann. Rev. Phys. Chem., Vol. 47, p. 421 (1996)
- Tanaka, 1992, Phase Transition of Gel
External links
States of matter Low energy High energy Other states Colloid · Glass · Liquid crystal · Magnetically ordered (Antiferromagnet, Ferrimagnet, Ferromagnet) · String-net liquid · SuperglassTransitions Boiling · Boiling point · Critical line · Critical point · Crystallization · Deposition · Evaporation · Flash evaporation · Freezing · Lambda point · Melting · Melting point · Regelation · Saturated fluid · Sublimation · Supercooling · Triple pointQuantities Enthalpy of fusion · Enthalpy of sublimation · Enthalpy of vaporization · Latent heat · Latent internal energy · Trouton's constant · Trouton's ratio · VolatilityConcepts Binodal · Compressed fluid · Cooling curve · Equation of state · Leidenfrost effect · Mpemba effect · Order and disorder (physics) · Spinodal · Superconductivity · Superheated vapor · Superheating · Thermo-dielectric effectRoutes of administration / Dosage forms Oral Buccal / Sublabial / Sublingual- Mouthwash
- Toothpaste
- Ointment
- Oral spray
- Oxygen mask
- Oxygen concentrator
- Anaesthetic machine
- Relative analgesia machine





Ocular / Otologic / Nasal - Nasal spray
- Ear drops
- Eye drops
- Ointment
- Hydrogel
- Nanosphere suspension
- Mucoadhesive microdisc (microsphere tablet)
Urogenital - Ointment
- Pessary (vaginal suppository)
- Vaginal ring
- Vaginal douche
- Intrauterine device (IUD)
- Extra-amniotic infusion
- Intravesical infusion
Rectal (enteral) - Ointment
- Suppository
- Enema (Solution • Hydrogel)
- Murphy drip
- Nutrient enema
Dermal Injection / Infusion
(into tissue/blood)- Intracavernous
- Intravitreal
- Intra-articular or intrasynovial injection
- Transscleral
- Intracerebral
- Intrathecal
- Epidural
Additional explanation: Mucous membranes are used by the human body to absorb the dosage for all routes of administration, except for "Dermal" and "Injection/Infusion".
Administration routes can also be grouped as Topical (local effect) or Systemic (defined as Enteral = Digestive tract/Rectal, or Parenteral = All other routes).Routes of administration by organ system Gastrointestinal Respiratory system Pulmonary • NasalVisual system / Auditory system Ocular (Ocular-topical / Intravitreal / Transscleral) • Otologic (Oto-topical)Reproductive system Intracavernous • Intravaginal • Intrauterine (Extra-amniotic)Urinary system IntravesicalPeritoneum Central nervous system Intracerebral • Intrathecal • EpiduralCirculatory system Musculoskeletal system Skin Epicutaneous • Intradermal • SubcutaneousCategories:- Chemical mixtures
- Colloidal chemistry
- Condensed matter physics
- Soft matter
- Dosage forms
Wikimedia Foundation. 2010.