- Gilman reagent
-
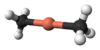
 General structure of a Gilman reagent
General structure of a Gilman reagent
A Gilman reagent is a lithium and copper (diorganocopper) reagent compound, R2CuLi, where R is an organic radical. These are useful because they react with organic chlorides, bromides, and iodides to replace the halide group with an R group. This is extremely useful in creating larger molecules from smaller ones.[1]
 Generalized chemical reaction showing Gilman reagent reacting with organic halide to form products and showing Cu(III) reaction intermediate
Generalized chemical reaction showing Gilman reagent reacting with organic halide to form products and showing Cu(III) reaction intermediate
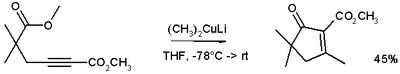
These reagents were discovered by Henry Gilman.[2] Lithium dimethylcopper (CH3)2CuLi can be prepared by adding copper(I) iodide to methyllithium in tetrahydrofuran at −78 °C. In the reaction depicted below,[3] the Gilman reagent is a methylating reagent reacting with an alkyne in a conjugate addition, and the negative charge is trapped in a nucleophilic acyl substitution with the ester group forming a cyclic enone.
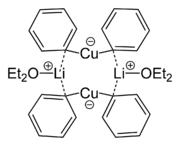
Gilman reagents have complicated structures in crystalline form and in solution. Lithium dimethylcuprate is a dimer in diethyl ether forming an 8-membered ring with two lithium atoms coordinating between two methyl groups. Similarly, lithium diphenylcuprate forms a dimeric etherate, [{Li(OEt2)}(CuPh2)]2, in the solid state.[4]
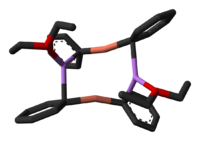
If the Li+ ions are rendered inert by complexation with the crown ether 12-crown-4, the isolated diorganylcuprate anions that remain adopt a linear coordination geometry at copper.[5]
See also
- Organolithium reagent
- Organocopper
- Grignard reagent
External links
References
- ^ J. F. Normant (1972). "Organocopper(I) Compounds and Organocuprates in Synthesis". Synthesis 1972 (02): 63–80. doi:10.1055/s-1972-21833.
- ^ Henry Gilman, Reuben G. Jones, and L. A. Woods (1952). "The Preparation of Methylcopper and some Observations on the Decomposition of Organocopper Compounds". Journal of Organic Chemistry 17 (12): 1630–1634. doi:10.1021/jo50012a009.
- ^ Modern Organocopper Chemistry, N. Krause Ed. Wiley-VCH, 2002.
- ^ N. P. Lorenzen, E. Weiss (1990). "Synthesis and Structure of a Dimeric Lithium Diphenylcuprate:[{Li(OEt)2}(CuPh2)]2". Angew. Chem. Int. Ed. 29 (3): 300–302. doi:10.1002/anie.199003001.
- ^ H. Hope, M. M. Olmstead, P. P. Power, J. Sandell, X. Xu (1985). "Isolation and x-ray crystal structures of the mononuclear cuprates [CuMe2]-, [CuPh2]-, and [Cu(Br)CH(SiMe3)2]-". J. Am. Chem. Soc. 107 (14): 4337–4338. doi:10.1021/ja00300a047.
Principles Reactions Types of compounds Applications Related branches of chemistry Categories:- Lithium compounds
- Reagents for organic chemistry
- Organocopper compounds
Wikimedia Foundation. 2010.