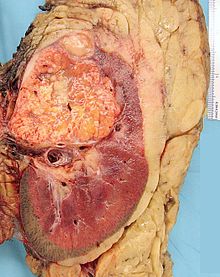
- Renal cell carcinoma
-
For other uses, see RCC (disambiguation).Main article: Kidney cancer
Renal cell carcinoma Classification and external resources 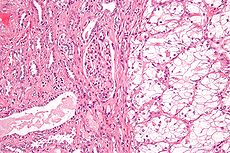
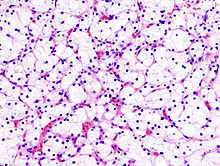
Micrograph of the most common type of renal cell carcinoma (clear cell) - on right of the image; non-tumour kidney is on the left of the image. Nephrectomy specimen. H&E stain.ICD-10 C64 ICD-9 189.0 ICD-O: M8312/3 OMIM 144700 605074 DiseasesDB 11245 MedlinePlus 000516 eMedicine med/2002 Renal cell carcinoma (RCC, also known as hypernephroma) is a kidney cancer that originates in the lining of the proximal convoluted tubule, the very small tubes in the kidney that filter the blood and remove waste products. RCC is the most common type of kidney cancer in adults, responsible for approximately 80% of cases.[1] It is also known to be the most lethal of all the genitourinary tumors. Initial treatment is most commonly a radical or partial nephrectomy and remains the mainstay of curative treatment.[2] Where the tumor is confined to the renal parenchyma, the 5-year survival rate is 60-70%, but this is lowered considerably where metastases have spread. It is resistant to radiation therapy and chemotherapy, although some cases respond to immunotherapy. Targeted cancer therapies such as sunitinib, temsirolimus, bevacizumab, interferon-alpha, and possibly sorafenib have improved the outlook for RCC (progression-free survival), although they have not yet demonstrated improved survival.
Contents
Signs and symptoms
A wide range of symptoms can be present with renal carcinoma depending on which areas of the body have been affected.[3] The classic triad is hematuria (blood in the urine), flank pain and an abdominal mass. This triad only occurs in 10-15% of cases, and is generally indicative of more advanced disease. Today, the majority of renal tumors are asymptomatic and are detected incidentally on imaging, usually for an unrelated cause.
Signs may include:
- abnormal urine color (dark, rusty, or brown) due to blood in the urine (found in 60% of cases)
- loin pain (found in 40% of cases)
- abdominal mass (25% of cases)
- malaise, weight loss or anorexia (30% of cases)[4]
- polycythemia (5% of cases)[4]
- anemia resulting from depression of erythropoietin (30% of cases)[4] Also, there may be erythrocytosis (increased production of red blood cells) due to increased erythropoietin secretion.[4]
- the presenting symptom may be due to metastatic disease, such as a pathologic fracture of the hip due to a metastasis to the bone
- varicocele, the enlargement of one testicle, usually on the left (2% of cases[5]). This is due to blockage of the left testicular vein by tumor invasion of the left renal vein; this typically does not occur on the right as the right gonadal vein drains directly into the inferior vena cava.
- vision abnormalities
- pallor or plethora
- hirsutism - excessive hair growth (females)
- constipation
- hypertension (high blood pressure) resulting from secretion of renin by the tumour (30% of cases)[4]
- elevated calcium levels (hypercalcemia)
- Stauffer syndrome - paraneoplastic, non-metastatic liver disease
- night sweats
- severe weight loss
Patients may also experience the following symptoms:
- recurrent fevers [6] which occur in 9% of the patients [7]
- cold intolerance [8]
- back pain
- chronic fatigue [9]
- leg and ankle swelling
- loss of appetite
Classification
Recent genetic studies have altered the approaches used in classifying renal cell carcinoma. The following system can be used to classify these tumors:[10][11][12]
- clear cell renal cell carcinoma (VHL, PBRM1 and others on chromosome 3)
- Papillary Renal Cell Carcinoma (MET, PRCC)
- chromophobe renal cell carcinoma
- collecting duct carcinoma
- clear cell papillary renal cell carcinoma
Renal epithelial neoplasms have characteristic cytogenetic aberrations that can aid in classification.[13] See also Atlas of Genetics and Cytogenetics in Oncology and Haematology.
- clear cell carcinoma: loss of 3p
- papillary carcinoma: trisomy 7, 16, 17
- chromophobe carcinoma: hypodiploid with loss of chromosomes 1, 2, 6, 10, 13, 17, 21
Array-based karyotyping can be used to identify characteristic chromosomal aberrations in renal tumors with challenging morphology.[14][15] Array-based karyotyping performs well on paraffin embedded tumors[16] and is amenable to routine clinical use. See also Virtual Karyotype for CLIA certified laboratories offering array-based karyotyping of solid tumors.
Other associated genes include TRC8, OGG1, HNF1A, HNF1B, TFE3, RCCP3, and RCC17.
Epidemiology
The incidence of renal cell cancer has been rising steadily. Nearly 51190 new diagnoses and 12890 deaths reported in the United States in 2007. It is more common in men than women: the male-to-female ratio is 1.6:1 and has been decreasing over the last decade. Blacks have a slightly higher rate of renal cell cancer than whites. The reasons for this are not clear.[17] Note: in epidemiology, RCC is registered together with renal pelvis carcinoma, which is predominantly transitional cell type.
In Europe the incidence of RCC has doubled in the period from 1975 to 2005.[18] RCC accounted for 3777 deaths in the UK in 2006; male 2372, female 1820.[19][20][21]
Risk factors
Cigarette smoking and obesity are the strongest known risk factors. Hypertension and a family history of the disease are also risk factors.[22] Occupational exposure to cadmium is a risk factor.
Dialysis patients with acquired cystic disease of the kidney showed a 30 times greater risk than in the general population for developing RCC.[23]
Exposure to asbestos, polycyclic aromatic hydrocarbons, gasoline has not been shown to be consistently associated with RCC risk.[24]
Patients with certain inherited disorders such as von Hippel-Lindau disease, hereditary papillary renal cancer, a hereditary leiomyoma RCC syndrome and Birt-Hogg-Dubé syndrome, show an enhanced risk of RCC.[25][26][27] Also, patients with sickle cell trait are predisposed to developing Renal medullary carcinoma.
Hysterectomy is associated with an approximately doubled risk. Hormonal factors or injury of the ureter during surgery were considered as possible causes.[28][29]
Diagnosis
An accurate diagnosis may be difficult to establish given that the early stages of renal cancer are asymptomatic.
The first steps taken in order to diagnose this condition are observing any of the signs and symptoms, and an anamnesis (the detailed medical review of past health state) to evaluate any risk factors. Upon physical examination, palpation of the abdomen may reveal the presence of a mass or an organ enlargement.[30]
However, the main diagnostic tool for detecting renal cell carcinoma is ultrasound of the kidneys.[31] If the ultrasound shows a mass or cyst, a subsequent abdominal CT is the optimal test for diagnosis and staging.[31]
Radiology
The characteristic appearance of renal cell carcinoma (RCC) is a solid renal lesion which disturbs the renal contour. It will frequently have an irregular or lobulated margin. Traditionally 85 to 90% of solid renal masses will turn out to be RCC but this number may be decreasing as renal masses are being found at smaller and smaller sizes with larger numbers of benign lesions. Ten percent of RCC will contain calcifications, and some contain macroscopic fat (likely due to invasion and encasement of the perirenal fat).
Renal cell carcinoma may also be cystic. As there are several benign cystic renal lesions (simple renal cyst, hemorrhagic renal cyst, multilocular cystic nephroma, polycystic kidney disease), it may occasionally be difficult for the radiologist to differentiate a benign cystic lesion from a malignant one. A classification system for cystic renal lesions that classifies them based specific imaging features into groups that are benign and those that need surgical resection is available.[32]
Percutaneous biopsy can be performed by a radiologist using ultrasound or computed tomography to guide sampling of the tumor for the purpose of diagnosis by pathology. However this is not routinely performed because when the typical imaging features of renal cell carcinoma are present, the possibility of an incorrectly negative result together with the risk of a medical complication to the patient make it unfavorable from a risk-benefit perspective. This is not completely accurate, there are new experimental treatments.
Staging
The staging of renal cell carcinoma is the most important factor in predicting its prognosis.[33] Staging can follow the TNM staging system, where the size and extent of the tumour (T), involvement of lymph nodes (N) and metastases (M) are classified separately. Also, it can use overall stage grouping into stage I-IV, with the 1997 revision of AJCC described below:[33]
Stage I Tumor of a diameter of 7 cm (approx. 23⁄4 inches) or smaller, and limited to the kidney. No lymph node involvement or metastases to distant organs. Stage II Tumor larger than 7.0 cm but still limited to the kidney. No lymph node involvement or metastases to distant organs. Stage III
any of the followingTumor of any size with involvement of a nearby lymph node but no metastases to distant organs. Tumor of this stage may be with or without spread to fatty tissue around the kidney, with or without spread into the large veins leading from the kidney to the heart. Tumor with spread to fatty tissue around the kidney and/or spread into the large veins leading from the kidney to the heart, but without spread to any lymph nodes or other organs. Stage IV
any of the followingTumor that has spread directly through the fatty tissue and the fascia ligament-like tissue that surrounds the kidney. Involvement of more than one lymph node near the kidney Involvement of any lymph node not near the kidney Distant metastases, such as in the lungs, bone, or brain. At diagnosis, 30% of renal cell carcinomas have spread to the ipsilateral renal vein, and 5-10% have continued into the inferior vena cava.[34]
Histopathology
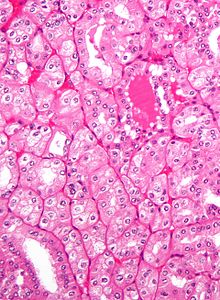
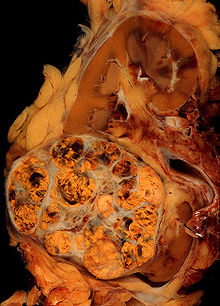
The gross and microscopic appearance of renal cell carcinomas is highly variable. The following describes a typical clear cell carcinoma, which is the most common type.
The renal cell carcinoma may present reddened areas where blood vessels have bled, and cysts containing watery fluids.[35] The body of the tumor shows large blood vessels that have walls composed of cancerous cells.
Gross examination often shows a yellowish, multilobulated tumor in the renal cortex, which frequently contains zones of necrosis, hemorrhage and scarring.
Light microscopy shows tumor cells forming cords, papillae, tubules or nests, and are atypical, polygonal and large. Also, the cells that make up a renal carcinoma may be clear, granular, mixed clear and granular or sarcomatoid or spindle type. Recent studies have brought to attention that the type of cancerous cells and the aggressiveness of the condition are closely related.Because these cells accumulate glycogen and lipids, their cytoplasm appear "clear", the nuclei remain in the middle of the cells, and the cellular membrane is evident. Some cells may be smaller, with eosinophilic cytoplasm, resembling normal tubular cells. The stroma is reduced, but well vascularized. The tumor compresses the surrounding parenchyma, producing a pseudocapsule.[36]
The clear cells are thought to be the least likely to spread and usually respond more favorably to treatment.[37] However, most of the tumors contain a mixture of cells. The most aggressive stage of renal cancer is believed to be the one in which the tumor is mixed, containing both clear and granular cells.
Prognosis
A study in Turkey that used the 1997 AJCC staging system estimated the five year survival rate to be 90% for stage I, 51% for stage II, 22% for stage III and 4.6% for stage IV.[38] The same study estimated the median survival time to be 7.7 years for stage I, 5.0 years for stage II, 3.1 years for stage III and 1.1 years for stage IV.[38]
For those that have tumor recurrence after surgery, the prognosis is generally poor. Renal cell carcinoma does not generally respond to chemotherapy or radiation. Immunotherapy, which attempts to induce the body to attack the remaining cancer cells, has shown promise. Recent trials are testing newer agents, though the current complete remission rate with these approaches are still low, around 12-20% in most series. Most recently, treatment with tyrosine kinase inhibitors including nexavar, pazopanib, and rapamycin have shown promise in improving the prognosis for advanced RCC since 2004.
Treatment
If it is only in the kidneys, which is about 40% of cases, it can be cured roughly 90% of the time with surgery. If it has spread outside of the kidneys, often into the lymph nodes or the main vein of the kidney, then it must be treated with adjunctive therapy, including cytoreductive surgery. RCC is resistant to chemotherapy and radiotherapy in most cases, but does respond well to immunotherapy with interleukin-2 or interferon-alpha, biologic, or targeted therapy. In early stage cases, cryotherapy and surgery are the preferred options.
Watchful waiting
Small renal tumors (< 4 cm) are treated increasingly by way of partial nephrectomy when possible.[39][40][41] Most of these small renal masses manifest indolent biological behavior with excellent prognosis.[42] More centers of excellence are incorporating needle biopsy to confirm the presence of malignant histology prior to recommending definitive surgical extirpation. In the elderly, patients with co-morbidities and in poor surgical candidates, small renal tumors may be monitored carefully with serial imaging. Most clinicians conservatively follow tumors up to a size threshold between 3 and 5 cm, beyond which the risk of distant spread (metastases) is about 5%.
Cryoablation
Cryoablation is used in a variety of clinical applications using hollow needles (cryoprobes) through which cooled, thermally conductive, fluids are circulated. Cryoprobes are inserted into or placed adjacent to tissue which is determined to be diseased in such a way that ablation will provide correction yielding benefit to the patient. When the probes are in place, the cryogenic freezing unit removes heat ("cools") from the tip of the probe and by extension from the surrounding tissues. The most common application of cryoablation is to ablate solid tumors found in the lung, liver, breast, kidney and prostate. The use in prostate and renal cryoablation are the most common. Although sometimes applied through laparoscopic or open surgical approaches, most often cryoablation is performed percutaneously (through the skin and into the target tissue containing the tumor).
Surgery
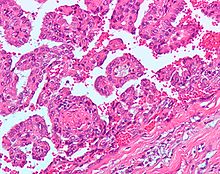
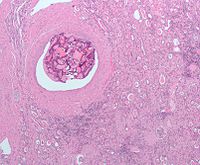 Micrograph of embolic material in a kidney removed because of renal cell carcinoma (cancer not shown). H&E stain.
Micrograph of embolic material in a kidney removed because of renal cell carcinoma (cancer not shown). H&E stain.
Surgical removal of all or part of the kidney (nephrectomy) is recommended.[2] This may include removal of the adrenal gland, retroperitoneal lymph nodes, and possibly tissues involved by direct extension (invasion) of the tumor into the surrounding tissues. In cases where the tumor has spread into the renal vein, inferior vena cava, and possibly the right atrium, this portion of the tumor can be surgically removed, as well. In cases of known metastases, surgical resection of the kidney ("cytoreductive nephrectomy") may improve survival,[43] as well as resection of a solitary metastatic lesion. Kidneys are sometimes embolized prior to surgery to minimize blood loss[1] (see image).
Surgery is increasingly performed via laparoscopic techniques. These have the advantage of being less of a burden for the patient and the disease-free survival is comparable to that of open surgery.[2] For small exophytic lesions that do not extensively involve the major vessels or urinary collecting system, a partial nephrectomy (also referred to as "nephron sparing surgery") can be performed. This may involve temporarily stopping blood flow to the kidney while the mass is removed as well as renal cooling with an ice slush. Mannitol can also be administered to help limit damage to the kidney. This is usually done through an open incision although smaller lesions can be done laparoscopically with or without robotic assistance.
Laparoscopic cryotherapy can also be done on smaller lesions. Typically a biopsy is taken at the time of treatment. Intraoperative ultrasound may be used to help guide placement of the freezing probes. Two freeze/thaw cycles are then performed to kill the tumor cells. As the tumor is not removed followup is more complicated (see below) and overall disease free rates are not as good as those obtained with surgical removal.
Percutaneous therapies
Percutaneous, image-guided therapies, usually managed by radiologists, are being offered to patients with localized tumor, but who are not good candidates for a surgical procedure. This sort of procedure involves placing a probe through the skin and into the tumor using real-time imaging of both the probe tip and the tumor by computed tomography, ultrasound, or even magnetic resonance imaging guidance, and then destroying the tumor with heat (radiofrequency ablation) or cold (cryotherapy). These modalities are at a disadvantage compared to traditional surgery in that pathologic confirmation of complete tumor destruction is not possible. Therefore, long-term follow-up is crucial to assess completeness of tumour ablation.[44][45]
Medications for advanced or metastatic cases
RCC "elicits an immune response, which occasionally results in dramatic spontaneous remissions." This has encouraged a strategy of using immunomodulating therapies, such as cancer vaccines and interleukin-2 (IL-2), to reproduce this response. IL-2 has produced "durable remissions" in a small number of patients, but with substantial toxicity. Another strategy is to restore the function of the VHL gene, which is to destroy proteins that promote inappropriate vascularization. Bevacizumab, an antibody to VEGF, has significantly prolonged time to progression, but As of 2008[update] phase 3 trials have not been published. Sunitinib (Sutent), sorafenib (Nexavar), and temsirolimus, which are small-molecule inhibitors of proteins, have been approved by the U.S. F.D.A.[46]
Treatment with tyrosine kinase inhibitors including Nexavar, pazopanib, and rapamycin have shown promise in improving the prognosis for advanced RCC since 2004.
Sorafenib (Nexavar), a protein kinase inhibitor, was FDA approved in December 2005 for treatment of advanced renal cell cancer.
A month later, Sunitinib (Sutent) was approved as well. Sunitinib (an oral, small-molecule, multi-targeted (RTK) inhibitor) and sorafenib both interfere with tumor growth by inhibiting angiogenesis as well as tumor cell proliferation. Sunitinib appears to offer greater potency against advanced RCC, perhaps because it inhibits more receptors than sorafenib.
2007 on
Temsirolimus (CCI-779) is an inhibitor of mTOR kinase (mammalian target of rapamycin) that was shown to prolong overall survival vs. interferon-α in patients with previously untreated metastatic renal cell carcinoma with three or more poor prognostic features. It was approved in May 2007 by the US FDA, and approved in EU in Nov 2007.
2007: Sunitinib; The first Phase III study comparing an RTKI with cytokine therapy was published in the New England Journal of Medicine. This study showed that Sunitinib offered superior efficacy compared with interferon-α. Progression-free survival (primary endpoint) was more than doubled. The benefit for sunitinib was significant across all major patient subgroups, including those with a poor prognosis at baseline. 28% of sunitinib patients had significant tumor shrinkage compared with only 5% of patients who received interferon-α. Although overall survival data are As of 2007[update] not yet mature, there is a clear trend toward improved survival with sunitinib. Patients receiving sunitinib also reported a significantly better quality of life than those treated with IFNa.[47]
June 2008: Good results were reported for Thalomid and Revlimid in trials for the treatment of renal cell carcinoma.[48]
March 2009 : Everolimus (Afinitor) (an oral once-daily inhibitor of mTOR) was approved by the US FDA for first treatment for patients with advanced kidney cancer after failure of either sunitinib or sorafenib.
2009: Carfilzomib, a novel proteasome inhibitor, shows efficacy and is well tolerated in relapsed RCC. [49]
In 2010 a Phase III trial of Axitinib for previously treated metastatic renal cell carcinoma (mRCC) showed significantly extended progression-free survival when compared to sorafenib.[50]
Chemotherapy
Most of the currently available cytostatics are ineffective for the treatment of RCC. Their use can not be recommended for the treatment of patients with metastasized RCC,as response rates are very low,often just 5-15%,and most responses are short lived.[1] The use of Tyrosine Kinase (TK) inhibitors, such as Sunitinib and Sorafenib, and Temsirolimus are described in a different section
Vaccine
Cancer vaccines, such as TroVax, have shown promising results in phase 2 trials for treatment of renal cell carcinoma.[51] However, issues of tumor immunosuppression and lack of identified tumor-associated antigens must be addressed before vaccine therapy can be applied successfully in advanced renal cell cancer.[52]
Metastatic renal cell carcinoma
The metastatic stage of renal cell carcinoma occurs when the disease invades and spreads to other organs. It is most likely to spread to neighboring lymph nodes, the lungs, the liver, the bones, or the brain.[53] Metastatic renal cell carcinoma presents a special challenge to oncologists, as about 70% of patients develop metastases during the course of their disease, and 5 year survival for patients with metastatic renal cell carcinoma is between 5 and 15%, although it is much improved if metastatectomy and nephrectomy to remove all visible disease is performed. Even if metastases are not removed, cytoreductive nephrectomy is sometimes used in the treatment of metastatic renal cell carcinoma, and at least one study has supported the use of this operation in "some cases", citing improved response rates to interleukin-2 immunotherapy and modestly prolonged survival.
Radiotherapy and chemotherapy have less of a role in the treatment of renal cell carcinoma than in other malignancies; but they are still sometimes used in treatment of the metastatic disease. Radiotherapy is used in in cases of bone metastases, to reduce pain and lower the risk of pathologic fracture, in patients with brain metastases, and to palliate symptoms of metastatic disease to the liver, adrenals, or lungs.
Interleukin-2 has been the standard of care since the 1990s in metastatic renal cell carcinoma, as, although response rates are low [7-16%], about half of patients that respond have long term disease-free survival, and some of these patients may be completely cured. However,the side effects of interleukin-2 are severe, including decreased neutrophil function, increased risk of disseminated infection, including central venous catheter infections, septicaemia, bacterial endocarditis, and capillary leak syndrome, which can result in myocardial infarction, renal failure, angina, hypotension, reduced organ perfusion, altered mental status, pulmonary failure requiring intubation, cardiac arrhythmias, edema, and gastrointestinal bleeding.
The use of proleukin can also result in lethargy and somnolence; if interleukin-2 therapy is not discontinued lethargy may progress to coma. Interleukin-2 can also worsen preexisting autoimmune diseases. Neurological side effects can also occur, and include ataxia, cortical blindness, hallucinations, psychosis, speech problems, and coma. Other side effects include abdominal pain, rigors, fever, malaise, asthenia, acidosis, tachycardia, vasodilatation, diarrhea, vomiting, mouth sores, loss of appetite, dermatitis, dyspnea, thrombocytopenia, and anaemia. Therefore, patients must be in good health with normal cardiovascular, hepatic, pulmonary,and neurological function to be treated with interleukin-2.
Recently, targeted therapies such as torisel, nexavar, sutent, votrient, and bevacizumab, have been developed, and all are now approved for the treatment of metastatic renal cell carcinoma. The three to five years up to 2009 saw dramatic improvements in treatment for those with metastatic renal cell carcinoma. However, despite these improvements in therapy, overall survival remains poor.
Currently[when?], tumor vaccines and chemotherapeutic, biologic, and immunologic agents are being researched in the treatment of metastatic renal cell carcinoma, and some appear promising. It is not known whether or not detecting metastases earlier improves survival or response to treatment.[citation needed]
Adjuvant therapy in renal cell carcinoma
Adjuvent therapy is typically a secondary treatment that is administered after all visible cancer has either been surgically excised, radiated or otherwise eliminated, in order to prevent any new (metastatic) cancer growths from re-appearing. The re-appearance of cancer typically occurs after micro-cancerous cells remain in the body after the primary cancer has been removed.
There is currently no established adjuvant therapy for renal cell carcinoma, although there have been a number of clinical trials exploring the effectiveness of various potential treatments.
The use of non-specific cytokines has so far been shown to be ineffective. Unlike most other cancers, renal cell carcinoma is resistant to most cytotoxic and cytostatic agents,which severely limits possible effective adjuvant therapy. Trials of "cancer vaccines", radiotherapy, chemotherapy, immunotherapy, or biologic therapies (i.e. nexavar, sutent) have been met with little success,and currently the standard of care for completely resected high-risk renal cell carcinoma is close observation with no other therapy. There does appear to be some evidence that if there cancer is incompletely resected (positive surgical margins,adrenal involvement,vena caval involvement) radiotherapy reduces the risk of invasive local disease,but data is lacking on that as well.[citation needed]
There have also been a number of trials of Autolymphocyte therapy (ALT) which have shown varying degrees of efficacy.[54][55][56][57][58]
ALT is a form of outpatient adoptive immunotherapy utilizing autologous ex vivo activated T-cells accompanied by high dose cimetidine.
History
Historically, RCC was also known as nephrocellular carcinoma.[59] Paul Grawitz first described renal cell carcinoma in 1883.
See also
- Stauffer syndrome
- Knudson hypothesis[60]
- interleukin-2
- kidney cancer
- rapamycin
- vinblastine
- dysuria
- interferon
References
- ^ a b c Mulders PF, Brouwers AH, Hulsbergen-van der Kaa CA, van Lin EN, Osanto S, de Mulder PH (February 2008). "[Guideline 'Renal cell carcinoma']" (in Dutch; Flemish). Ned Tijdschr Geneeskd 152 (7): 376–80. PMID 18380384.
- ^ a b c Rini BI, Rathmell WK, Godley P (May 2008). "Renal cell carcinoma". Curr Opin Oncol 20 (3): 300–6. doi:10.1097/CCO.0b013e3282f9782b. PMID 18391630.
- ^ "Renal Carcinoma or hypernephroma". http://www.renalcarcinoma.net/. Retrieved 2010-03-31.
- ^ a b c d e Kumar, Parveen & Clark, Michael, eds (2005). Clinical Medicine (Sixth ed.). London: Elsevier. pp. 683–648. ISBN 0-7020-2763-4.
- ^ Motzer RJ, Bander NH, Nanus DM (September 1996). "Renal-cell carcinoma". N. Engl. J. Med. 335 (12): 865–75. doi:10.1056/NEJM199609193351207. PMID 8778606. http://content.nejm.org/cgi/pmidlookup?view=short&pmid=8778606&promo=ONFLNS19.
- ^ "Renal Cell Cancer". http://www.cancercompass.com/renal-cell-cancer-information/symptoms.htm/. Retrieved 2010-03-31.
- ^ "Renal Cell Carcinoma". http://www.omnimedicalsearch.com/conditions-diseases/renal-cell-carcinoma-symptoms.html. Retrieved 2010-03-31.
- ^ "Causes". http://www.nlm.nih.gov/medlineplus/ency/article/000516.htm. Retrieved 2010-03-31.
- ^ "Kidney Cancer Symptoms (Renal Cell)". http://cancer.about.com/od/kidneycancerrenalcell/a/kidneysymptoms.htm. Retrieved 2010-03-31.
- ^ Reuter VE, Presti JC (April 2000). "Contemporary approach to the classification of renal epithelial tumors". Semin. Oncol. 27 (2): 124–37. PMID 10768592.
- ^ Bodmer D, van den Hurk W, van Groningen JJ, et al. (October 2002). "Understanding familial and non-familial renal cell cancer". Hum. Mol. Genet. 11 (20): 2489–98. doi:10.1093/hmg/11.20.2489. PMID 12351585. http://hmg.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=12351585.
- ^ Cotran, Ramzi S.; Kumar, Vinay; Fausto, Nelson; Nelso Fausto; Robbins, Stanley L.; Abbas, Abul K. (2005). Robbins and Cotran pathologic basis of disease. St. Louis, Mo: Elsevier Saunders. p. 1016. ISBN 0-7216-0187-1.
- ^ van den Berg E, Storkel S . Kidney: Renal cell carcinoma. Atlas Genet Cytogenet Oncol Haematol. June 2003.
- ^ Hagenkord JM, Parwani AV, Lyons-Weiler MA, et al. (2008). "Virtual karyotyping with SNP microarrays reduces uncertainty in the diagnosis of renal epithelial tumors". Diagn Pathol 3: 44. doi:10.1186/1746-1596-3-44. PMC 2588560. PMID 18990225. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2588560.
- ^ Monzon FA, Hagenkord JM, Lyons-Weiler MA, et al. (May 2008). "Whole genome SNP arrays as a potential diagnostic tool for the detection of characteristic chromosomal aberrations in renal epithelial tumors". Mod. Pathol. 21 (5): 599–608. doi:10.1038/modpathol.2008.20. PMID 18246049.
- ^ Lyons-Weiler M, Hagenkord J, Sciulli C, Dhir R, Monzon FA (March 2008). "Optimization of the Affymetrix GeneChip Mapping 10K 2.0 Assay for routine clinical use on formalin-fixed paraffin-embedded tissues". Diagn. Mol. Pathol. 17 (1): 3–13. doi:10.1097/PDM.0b013e31815aca30. PMID 18303412.
- ^ American Cancer Society. Cancer facts & figures 2007. American Cancer Society; 2007.
- ^ Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P (March 2007). "Estimates of the cancer incidence and mortality in Europe in 2006". Ann. Oncol. 18 (3): 581–92. doi:10.1093/annonc/mdl498. PMID 17287242.
- ^ Office for National Statistics, Mortality Statistics: Cause England & Wales, 2006. Vol. DH2 No.32. 2006: TSO.
- ^ GRO for Scotland Registrar General's Annual Report, 2006.
- ^ Northern Ireland Cancer Registry, Cancer Mortality in Northern Ireland, 2006. 2006.
- ^ Lipworth L, Tarone RE, McLaughlin JK (December 2006). "The epidemiology of renal cell carcinoma". J. Urol. 176 (6 Pt 1): 2353–8. doi:10.1016/j.juro.2006.07.130. PMID 17085101.
- ^ Brennan JF, Stilmant MM, Babayan RK, Siroky MB (April 1991). "Acquired renal cystic disease: implications for the urologist". Br J Urol 67 (4): 342–8. doi:10.1111/j.1464-410X.1991.tb15158.x. PMID 2032071.
- ^ McLaughlin, J. K. (2006). Kidney Cancer. Oxford: Oxford University Press. pp. 1087–1100.
- ^ Lamiell JM, Salazar FG, Hsia YE (January 1989). "von Hippel-Lindau disease affecting 43 members of a single kindred". Medicine (Baltimore) 68 (1): 1–29. PMID 2642584.
- ^ Pavlovich CP, Schmidt LS (May 2004). "Searching for the hereditary causes of renal-cell carcinoma". Nat. Rev. Cancer 4 (5): 381–93. doi:10.1038/nrc1364. PMID 15122209.
- ^ Washecka R, Hanna M (April 1991). "Malignant renal tumors in tuberous sclerosis". Urology 37 (4): 340–3. doi:10.1016/0090-4295(91)80261-5. PMID 2014599.
- ^ Gago-Dominguez, M.; Castelao, J. E.; Yuan, J. M.; Ross, R. K.; Yu, M. C. (1999). "Increased risk of renal cell carcinoma subsequent to hysterectomy". Cancer Epidemiology, Biomarkers & Prevention 8 (11): 999–1003. PMID 10566555.
- ^ Zucchetto, A.; Talamini, R.; Dal Maso, L.; Negri, E.; Polesel, J.; Ramazzotti, V.; Montella, M.; Canzonieri, V. et al. (2008). "Reproductive, menstrual, and other hormone-related factors and risk of renal cell cancer". International Journal of Cancer 123 (9): 2213. doi:10.1002/ijc.23750. PMID 18711701.
- ^ "Renal cell carcinoma". http://www.nlm.nih.gov/medlineplus/ency/article/000516.htm/. Retrieved 2010-03-31.[dead link]
- ^ a b Elizabeth D Agabegi; Agabegi, Steven S. (2008). Step-Up to Medicine (Step-Up Series). Hagerstwon, MD: Lippincott Williams & Wilkins. ISBN 0-7817-7153-6.
- ^ Israel GM, Bosniak MA (August 2005). "How I do it: evaluating renal masses". Radiology 236 (2): 441–50. doi:10.1148/radiol.2362040218. PMID 16040900.
- ^ a b Kidney Cancer / General Information at Weill Cornell Medical College, James Buchanan Brady Foundation, Department of Urology
- ^ Oto A, Herts BR, Remer EM, Novick AC (December 1998). "Inferior vena cava tumor thrombus in renal cell carcinoma: staging by MR imaging and impact on surgical treatment". AJR Am J Roentgenol. 171 (6): 1619–24. PMID 9843299. http://www.ajronline.org/cgi/pmidlookup?view=long&pmid=9843299.
- ^ "Clear-cell Carcinoma, Hypernephroid Tumour, or Hypernephroma". http://www.britannica.com/EBchecked/topic/497921/renal-carcinoma. Retrieved 2010-03-31.
- ^ "pathologyatlas.ro". http://www.pathologyatlas.ro/renal-cell-carcinoma-grawitz-tumor-kidney-pathology.php. Retrieved 2007-12-29.
- ^ "PathologyPathology". http://www.urologychannel.com/kidneycancer/pathology.shtml. Retrieved 2010-03-31.
- ^ a b Erdo�an, F.; Demirel, A.; Polat, Ö. (2004). "Prognostic significance of morphologic parameters in renal cell carcinoma". International Journal of Clinical Practice 58 (4): 333–336. doi:10.1111/j.1368-5031.2004.00008.x. PMID 15161115.
- ^ Novick AC (September 1998). "Nephron-sparing surgery for renal cell carcinoma". Br J Urol 82 (3): 321–4. doi:10.1046/j.1464-410X.1998.00751.x. PMID 9772865.
- ^ Herr HW (January 1999). "Partial nephrectomy for unilateral renal carcinoma and a normal contralateral kidney: 10-year followup". J. Urol. 161 (1): 33–4; discussion 34–5. doi:10.1016/S0022-5347(01)62052-4. PMID 10037361.
- ^ Van Poppel H, Bamelis B, Oyen R, Baert L (September 1998). "Partial nephrectomy for renal cell carcinoma can achieve long-term tumor control". J. Urol. 160 (3 Pt 1): 674–8. doi:10.1016/S0022-5347(01)62751-4. PMID 9720519.
- ^ Mattar K, Jewett MA (January 2008). "Watchful waiting for small renal masses". Curr Urol Rep 9 (1): 22–5. doi:10.1007/s11934-008-0006-3. PMID 18366970.
- ^ Flanigan RC, Mickisch G, Sylvester R, Tangen C, Van Poppel H, Crawford ED (March 2004). "Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis". J Urol. 171 (3): 1071–6. doi:10.1097/01.ju.0000110610.61545.ae. PMID 14767273.
- ^ Mogami T, Harada J, Kishimoto K, Sumida S (April 2007). "Percutaneous MR-guided cryoablation for malignancies, with a focus on renal cell carcinoma". Int. J. Clin. Oncol. 12 (2): 79–84. doi:10.1007/s10147-006-0654-6. PMID 17443274.
- ^ Boss A, Clasen S, Kuczyk M, Schick F, Pereira PL (March 2007). "Image-guided radiofrequency ablation of renal cell carcinoma". Eur Radiol 17 (3): 725–33. doi:10.1007/s00330-006-0415-y. PMID 17021704.
- ^ Michaelson MD, Iliopoulos O, McDermott DF, McGovern FJ, Harisinghani MG, Oliva E (May 2008). "Case records of the Massachusetts General Hospital. Case 17-2008. A 63-year-old man with metastatic renal-cell carcinoma". N Engl J Med. 358 (22): 2389–96. doi:10.1056/NEJMcpc0802449. PMID 18509125. http://content.nejm.org/cgi/content/full/358/22/2389. Needs NEJM subscription
- ^ Motzer RJ et al. (2007). "Sunitinib versus interferon alfa in metastatic renal-cell carcinoma". N Engl J Med 356 (2): 115–124. doi:10.1056/NEJMoa065044. PMID 17215529.
- ^ "Thalomid and Revlimid Active as Primary Treatment for Metastatic Renal Cell Carcinoma". June 2008. http://professional.cancerconsultants.com/oncology_renal_cancer_news.aspx?id=42229.
- ^ http://www.asco.org/ascov2/Meetings/Abstracts?&vmview=abst_detail_view&confID=65&abstractID=33781
- ^ "Pfizer’s Phase III Trial in mRCC Turns Up Positive Results". 19 Nov 2010. http://www.genengnews.com/gen-news-highlights/pfizer-s-phase-iii-trial-in-mrcc-turns-up-positive-results/81244271/.
- ^ "Vaccine for kidney and bowel cancers 'within three years'". Daily Mail (London). 2006-11-13. http://www.dailymail.co.uk/pages/live/articles/news/news.html?in_article_id=416006&in_page_id=1770.
- ^ Amato RJ (September 2008). "Vaccine therapy for renal cancer". Expert Rev Vaccines 7 (7): 925–35. doi:10.1586/14760584.7.7.925. PMID 18767943.
- ^ "Renal Cell Cancer". http://www.emedicinehealth.com/renal_cell_cancer/article_em.htm/. Retrieved 2010-03-31.
- ^ Randomized, controlled trial of adjuvant therapy with ex vivo activated T cells (ALT) in T1-3a,b,c or T4N+,M 0 renal cell carcinoma'
- ^ Biotherapy of advanced cancer by infusion of in vitro activated'
- ^ x vivo activated memory T-lymphocytes as adoptive cellular therapy of human renal cell tumour targets with potentiation by cis-diamminedichloroplatinum(II).'
- ^ Treatment of metastatic renal cell carcinoma with autolymphocyte therapy'
- ^ Effect of autolymphocyte therapy on survival and quality of life in patients'
- ^ Vasil'eva NN (1975). "[Patho-anatomical characteristics of renal cell cancer]" (in Russian). Arkh. Patol. 37 (10): 11–8. PMID 1225265.
- ^ Valladares Ayerbes M, Aparicio Gallego G, Díaz Prado S, Jiménez Fonseca P, García Campelo R, Antón Aparicio LM (November 2008). "Origin of renal cell carcinomas". Clin Transl Oncol 10 (11): 697–712. doi:10.1007/s12094-008-0276-8. PMID 19015066.
Tumors: urogenital neoplasia: urinary organs (C64–C68/D30, 188–189/223) Abdominal KidneyGlandular and epithelial neoplasm: Renal cell carcinoma · Renal oncocytomaUreterPelvic Retroperitoneum Glandular and epithelial neoplasms (ICD-O 8010-8589) Epithelium Small cell carcinoma · Combined small cell carcinoma · Verrucous carcinoma · Squamous cell carcinoma · Basal cell carcinoma · Transitional cell carcinoma · Inverted papillomaGlands Other/multipleAdnexal and
skin appendage (8390-8429)Cystic, mucinous,
and serous (8440-8499)Cystic generalSerousOvarian serous cystadenoma/Pancreatic serous cystadenoma/Serous cystadenocarcinoma/Papillary serous cystadenocarcinomaDuctal, lobular,
and medullary (8500-8549)Lobular carcinoma in situ · Invasive lobular carcinomaAcinar cell (8550-8559)Other Complex epithelial (8560-8589)see also Template:Epithelium and epithelial tissueCategories:
Wikimedia Foundation. 2010.