- Isotope separation
-
Isotope separation is the process of concentrating specific isotopes of a chemical element by removing other isotopes, for example separating natural uranium into enriched uranium and depleted uranium. This is a crucial process in the manufacture of uranium fuel for nuclear power stations, and is also required for the creation of uranium based nuclear weapons. Plutonium based weapons use plutonium produced in a nuclear reactor, which must be operated in such a way as to produce plutonium already of suitable isotopic mix or grade. This theory was first recognized by Charles H. Townes. While in general chemical elements can be purified through chemical processes, isotopes of the same element have nearly identical chemical properties, which makes this type of separation impractical, except for separation of deuterium.
Contents
Separation techniques
There are three types of isotope separation techniques:
- Those based directly on the atomic weight of the isotope.
- Those based on the small differences in chemical reaction rates produced by different atomic weights.
- Those based on properties not directly connected to atomic weight, such as nuclear resonances.
The third type of separation is still experimental; practical separation techniques all depend in some way on the atomic mass. It is therefore generally easier to separate isotopes with a larger relative mass difference. For example deuterium has twice the mass of ordinary (light) hydrogen and it is generally easier to purify it than to separate uranium-235 from the more common uranium-238. On the other extreme, separation of fissile plutonium-239 from the common impurity plutonium-240, while desirable in that it would allow the creation of gun-type nuclear weapons from plutonium, is generally agreed to be impractical.[citation needed]
- See also: Enriched uranium
Enrichment cascades
All large-scale isotope separation schemes employ a number of similar stages which produce successively higher concentrations of the desired isotope. Each stage enriches the product of the previous step further before being sent to the next stage. Similarly, the tailings from each stage are returned to the previous stage for further processing. This creates a sequential enriching system called a cascade.
There are two important factors that affect the performance of a cascade. The first is the separation factor (the square root of the mass ratio of the two isotopes), which is a number greater than 1. The second is the number of required stages to get the desired purity.
Commercial materials
To date, large-scale commercial isotope separation of only three elements has occurred. In each case, the rarer of the two most common isotopes of an element has been concentrated for use in nuclear technology:
- Uranium isotopes have been separated to prepare enriched uranium for use as nuclear reactor fuel and in nuclear weapons.
- Hydrogen isotopes have been separated to prepare heavy water for use as a moderator in nuclear reactors.
- Lithium-6 has been concentrated for use in thermonuclear weapons.
Some isotopically purified elements are used in smaller quantities for specialist applications, especially in the semiconductor industry, where purified Silicon is used to improve crystal structure and thermal conductivity.[1]
Isotope separation is an important process for both peaceful and military nuclear technology, and therefore the capability that a nation has for isotope separation is of extreme interest to the intelligence community.
Alternatives
The only alternative to isotope separation is to manufacture the required isotope in its pure form. This may be done by irradiation of a suitable target, but care is needed in target selection and other factors to ensure that only the required isotope of the element of interest is produced. Isotopes of other elements are not so great a problem as they can be removed by chemical means.
This is particularly relevant in the preparation of high-grade plutonium-239 for use in weapons. It is not practical to separate Pu-239 from Pu-240 or Pu-241. Fissile Pu-239 is produced following neutron capture by uranium-238, but further neutron capture will produce non-fissile Pu-240 and worse, then Pu-241 which is a fairly strong neutron emitter. Therefore, the uranium targets used to produce military plutonium must be irradiated for only a short time, to minimise the production of these unwanted isotopes. Conversely, blending plutonium with Pu-241 renders it unsuitable for nuclear weapons.
Practical methods of separation
Diffusion
Often done with gases, but also with liquids, the diffusion method relies on the fact that in thermal equilibrium, two isotopes with the same energy will have different average velocities. The lighter atoms (or the molecules containing them) will travel more quickly and be more likely to diffuse through a membrane. The difference in speeds is proportional to the square root of the mass ratio, so the amount of separation is small and many cascaded stages are needed to obtain high purity. This method is expensive due to the work needed to push gas through a membrane and the many stages necessary.
The first large-scale separation of uranium isotopes was achieved by the United States in large gaseous diffusion separation plants at Oak Ridge Laboratories, which were established as part of the Manhattan Project. These used uranium hexafluoride gas as the process fluid. Nickel powder and electro-deposited nickel mesh diffusion barriers were pioneered by Edward Adler and Edward Norris.[2] See gaseous diffusion.
Centrifugal effect
Centrifugal effect schemes rapidly rotate the material allowing the heavier isotopes to go closer to an outer radial wall. This too is often done in gaseous form using a Zippe-type centrifuge.
The centrifugal separation of isotopes was first suggested by Aston and Lindemann[3] in 1919 and the first successful experiments were reported by Beams and Haynes[4] on isotopes of chlorine in 1936. However attempts to use the technology during the Manhattan project were unproductive. In modern times it is the main method used throughout the world to enrich uranium and as a result remains a fairly secretive process, hindering a more widespread uptake of the technology. In general a feed of UF6 gas is connected to a cylinder that is rotated at high speed. Near the outer edge of the cylinder heavier gas molecules containing U-238 collect, while molecules containing U-235 concentrate at the center and are then fed to another cascade stage.[5] Use of gaseous centrifugal technology to enrich isotopes is desirable as power consumption is greatly reduced when compared to more conventional techniques such as diffusion plants since fewer cascade steps are required to reach similar degrees of separation. In fact, Gas centrifuges using uranium hexafluoride have largely replaced gaseous diffusion technology for uranium enrichment.[citation needed] As well as requiring less energy to achieve the same separation, far smaller scale plants are possible, making them an economic possibility for a small nation attempting to produce a nuclear weapon. Pakistan is believed to have used this method in developing its nuclear weapons.
Vortex tubes were used by South Africa in their Helikon vortex separation process. The gas is injected tangentially into a chamber with special geometry that further increases its rotation to a very high rate, causing the isotopes to separate. The method is simple because vortex tubes have no moving parts, but energy intensive, about 50 times greater than gas centrifuges. A similar process, known as jet nozzle, was created in Germany, with a demonstration plant built in Brazil, and they went as far as developing a site to fuel the country's nuclear plants.
Electromagnetic
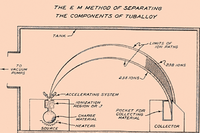 Schematic diagram of uranium isotope separation in a calutron.
Schematic diagram of uranium isotope separation in a calutron.
This method is a form of mass spectrometry, and is sometimes referred to by that name. It uses the fact that charged particles are deflected in a magnetic field and the amount of deflection depends upon the particle's mass. It is very expensive for the quantity produced, as it has an extremely low throughput, but it can allow very high purities to be achieved. This method is often used for processing small amounts of pure isotopes for research or specific use (such as isotopic tracers), but is impractical for industrial use.
At Oak Ridge and at the University of California, Berkeley, Ernest O. Lawrence developed electromagnetic separation for much of the uranium used in the first United States atomic bomb (see Manhattan Project). Devices using his principle are named calutrons. After the war the method was largely abandoned as impractical. It had only been undertaken (along with diffusion and other technologies) to guarantee there would be enough material for use, whatever the cost. Its main eventual contribution to the war effort was to further concentrate material from the gaseous diffusion plants to even higher levels of purity.
Laser
In this method a laser is tuned to a wavelength which excites only one isotope of the material and ionizes those atoms preferentially. The resonant absorption of light for an isotope is dependent upon its mass and certain hyperfine interactions between electrons and the nucleus, allowing finely tuned lasers to interact with only one isotope. After the atom is ionized it can be removed from the sample by applying an electric field. This method is often abbreviated as AVLIS (atomic vapor laser isotope separation). This method has only recently been developed as laser technology has improved, and is currently not used extensively. However, it is a major concern to those in the field of nuclear proliferation because it may be cheaper and more easily hidden than other methods of isotope separation. Tunable lasers used in AVLIS include the dye laser [6] and more recently diode lasers.[7]
A second method of laser separation is known as MLIS, Molecular Laser Isotope Separation. In this method, an infrared laser is directed at uranium hexafluoride gas, exciting molecules that contain a U-235 atom. A second laser frees a fluorine atom, leaving uranium pentafluoride which then precipitates out of the gas. Cascading the MLIS stages is more difficult than with other methods because the UF5 must be refluorinated (back to UF6) before being introduced into the next MLIS stage. Alternative MLIS schemes are currently being developed (using a first laser in the near-infrared or visible region) where an enrichment of over 95% can be obtained in a single stage, but the methods have not (yet) reached industrial feasibility. This method is called OP-IRMPD (Overtone Pre-excitation - IR Multiple Photon Dissociation).
Finally, the SILEX process, developed by Silex Systems in Australia, has recently been licensed to General Electric for the development of a pilot enrichment plant. The method uses uranium hexafluoride as a feedstock, and uses magnets to separate the isotopes after one isotope is preferentially ionized. Further details of the process are not disclosed.
Chemical methods
Although isotopes of a single element are normally described as having the same chemical properties, this is not strictly true. In particular, reaction rates are very slightly affected by atomic mass.
Techniques using this are most effective for light atoms such as hydrogen. Lighter isotopes tend to react or evaporate more quickly than heavy isotopes, allowing them to be separated. This is how heavy water is produced commercially, see Girdler sulfide process for details. Lighter isotopes also disassociate more rapidly under an electric field. This process in a large cascade was used at the heavy water production plant at Rjukan.
One candidate for the largest kinetic isotopic effect ever measured at room temperature, 305, may eventually be used for the separation of tritium (T). The effects for the oxidation of triated formate anions to HTO were measured as:
k(HCO2-) = 9.54 M−1s−1 k(H)/k(D) = 38 k(DCO2-) = 9.54 M−1s−1 k(D)/k(T) = 8.1 k(TCO2-) = 9.54 M−1s−1 k(H)/k(T) = 305 Gravity
Isotopes of Carbon, Oxygen, and Nitrogen can be purified by chilling these gases or compounds nearly to their liquification temperature in very tall columns (200 to 700 feet tall - 70 to 200 meters). The heavier isotopes sink and the lighter isotopes rise, where they are easily collected. The process was developed in the late 1960s by scientists at Los Alamos National Laboratory.[1] This process is also called "cryogenic distillation".[2]
The SWU (separative work unit)
Separative Work Unit (SWU) is a complex unit which is a function of the amount of uranium processed and the degree to which it is enriched, i.e. the extent of increase in the concentration of the U-235 isotope relative to the remainder.
The unit is strictly: Kilogram Separative Work Unit, and it measures the quantity of separative work (indicative of energy used in enrichment) when feed and product quantities are expressed in kilograms. The effort expended in separating a mass F of feed of assay xf into a mass P of product assay xp and waste of mass W and assay xw is expressed in terms of the number of separative work units needed, given by the expression SWU = WV(xw) + PV(xp) - FV(xf), where V(x) is the "value function," defined as V(x) = (1 - 2x) ln ((1 - x) /x).
Separative work is expressed in SWUs, kg SW, or kg UTA (from the German Urantrennarbeit )
- 1 SWU = 1 kg SW = 1 kg UTA
- 1 kSWU = 1.0 t SW = 1 t UTA
- 1 MSWU = 1 kt SW = 1 kt UTA
If, for example, you begin with 100 kilograms (220 pounds) of natural uranium, it takes about 60 SWU to produce 10 kilograms (22 pounds) of uranium enriched in U-235 content to 4.5%
Radioactive beams of specific isotopes are widely used in the fields of experimental physics, biology and materials science. The production and formation of these radioactive atoms into an ionic beam for study is an entire field of research carried out at many laboratories throughout the world. The first isotope separator was developed at the Copenhagen Cyclotron by Bohr and co-workers using the principle of electromagnetic separation. Today, there are many laboratories around the world which supply beams of radioactive ions for use. Arguably the principal Isotope Separator On-Line (ISOL) is ISOLDE at CERN, [3] which is a joint European facility spread across the Franco-Swiss border near the city of Geneva. This laboratory uses mainly proton spallation of uranium carbide targets to produce a wide range of radioactive fission fragments that are not found naturally on earth. During spallation (bombardment with high energy protons), a uranium carbide target is heated to several thousand degrees so that radioactive atoms produced in the nuclear reaction are released. Once out of the target, the vapour of radioactive atoms travels to an ionizer cavity. This ionizer cavity is a thin tube made of a low work function metal allowing for collisions with the walls to liberate a single electron from a free atom. Once ionized, the radioactive species are accelerated by an electrostatic field and injected into an electromagnetic separator. As ions entering the separator are of approxiamtely equal energy, those ions with a smaller mass will be deflected by the magnetic field by a greater amount than those with a heavier mass. This differing radius of curvature allows for isobaric purification to take place. Once purified isobarically, the ion beam is then sent to the individual experiments. In order to increase the purity of the isobaric beam, laser ionization can take place inside the ionizer cavity to selectively ionize a single element chain of interest. At CERN, this device is called the Resonance Ionization Laser Ion Source (RILIS). Currently over 60% of all experiments opt to use the RILIS to increase the purity of radioactive beams.
Beam Production Capability of ISOL Facilities
As the production of radioactive atoms by the ISOL technique depends on the free atom chemistry of the element to be studied, there are certain beams which cannot be produced by simple proton bombardment of thick actinide targets. Refractory metals such as tungsten and rhenium do not emerge from the target even at high temperatures due to their low vapour pressure. In order to produce these types of beams, a thin target is required. The Ion Guide Isotope Separator On Line (IGISOL) technique was developed in 1981 at the University of Jyvaskyla cyclotron laboratory in Finland [4]. In this technique, a thin uranium target is bombarded with protons and nuclear reaction products recoil out of the target in a charged state. The recoils are stopped in a gas cell and then exit through a small hole in the side of the cell where they are accelerated electrostatically and injected into a mass separator. This method of production and extraction takes place on a shorter timescale compared to the standard ISOL technique and isotopes with short half-lives (sub millisecond) can be studied using an IGISOL. An IGISOL has also been combined with a laser ion source at the Leuven Isotope Separator On Line (LISOL) in Belgium [5]. Thin target sources generally provide significantly lower quantities of radioactive ions than thick target sources and this is their main drawback.
As experimental nuclear physics progresses, it is becoming more and more important to study the most exotic of radioactive nuclei. In order to do so, more inventive techniques are required to create nuclei with extreme proton/neutron ratios. The most promising technique to date is by using multiple targets. By first producing a radioactive beam by an ISOL method and then reaccellerating it to make it hit a secondary thin target, very exotic nuclei can be produced. The National Superconducting Cyclotron Laboratory (NSCL) at Michigan State University is a good example of such a facility. The higher the energy of interaction, generally the more exotic the nucleus produced. It then becomes necessary to be able to slow these nuclei down once they have been produced. Pioneers at the Japanese facility RIKEN were the first to use a giant gas catcher and novel electric fields to do this, which is becoming the standard technique.
References
- ^ http://www.theregister.co.uk/2000/11/30/amd_tests_super_silicon/
- ^ Rhodes, Richard, The Making of the Atomic Bomb, 1986, p. 494.
- ^ F. A. Lindemann and F. W. Aston, The possibility of separating isotopes, Philos. Mag., 1919, 37, p. 523.
- ^ J. W. Beams and F. B. Haynes, The Separation of Isotopes by Centrifuging, Phys. Rev., 1936, 50, pp. 491-492.
- ^ Stanley Whitley, Review of the gas centrifuge until 1962. Part I: Principles of separation physics, Rev. Mod. Phys., 1984, 56, pp. 41-66.
- ^ F. J. Duarte and L.W. Hillman (Eds.), Dye Laser Principles (Academic, New York, 1990) Chapter 9.
- ^ F. J. Duarte (Ed.), Tunable Laser Applications, 2nd Ed. (CRC, 2008) Chapter 11
External links
- Utilization of kinetic isotope effects for the concentration of tritium, GM Brown, TJ Meyer et al., 2001.
- Uranium Production
- Uranium Enrichment from the World Nuclear Association
- Annotated bibliography on electromagnetic separation of uranium isotopes form the Alsos Digital Library
Categories:
Wikimedia Foundation. 2010.

