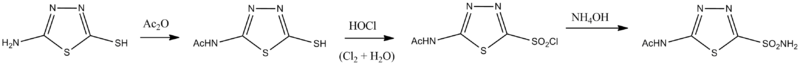- Acetazolamide
-
Not to be confused with acetohexamide.
Acetazolamide 

Systematic (IUPAC) name N-(5-sulfamoyl-1,3,4-thiadiazol-2-yl)acetamide Clinical data Trade names Diamox AHFS/Drugs.com monograph Pregnancy cat. B3(AU) C(US) Legal status POM (UK) ℞-only (US) Routes Oral, IV Pharmacokinetic data Metabolism None Half-life 3 to 9 hours Excretion Renal Identifiers CAS number 59-66-5 
ATC code S01EC01 PubChem CID 1986 DrugBank DB00819 ChemSpider 1909 
UNII O3FX965V0I 
KEGG D00218 
ChEBI CHEBI:27690 
ChEMBL CHEMBL20 
Chemical data Formula C4H6N4O3S2 Mol. mass 222.245 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Acetazolamide, sold under the trade name Diamox, is a carbonic anhydrase inhibitor that is used to treat glaucoma, epileptic seizures, Idiopathic intracranial hypertension (pseudotumor cerebri), altitude sickness, cystinuria, and dural ectasia. Acetazolamide is available as a generic drug and is also a diuretic.
Contents
Mechanism of action
Acetazolamide is a carbonic anhydrase inhibitor. Medically it may be used to treat conditions of moderate to severe metabolic or respiratory alkalosis. It does this by interfering with bicarbonate (HCO3-) resorption in the kidneys, thereby re-acidifying the blood (and thus alkalinizing the urine).
Carbonic anhydrase (CA) catalyzes the first part of the following reversible reaction, in which carbon dioxide (CO2) and water (H2O) are converted to carbonic acid (H2CO3) and vice-versa:
CO2 + H2O <--CA--> H2CO3 <--> H+ + HCO3-
In the kidney tubules, locally secreted hydrogen ions normally combine with filtered bicarbonate (HCO3-) to form carbonic acid (H2CO3). Carbonic acid in turn is normally acted upon by carbonic anhydrase, leading to formation of CO2. As CO2 rapidly leaves the tubules by diffusing across cell membranes, the above reaction normally runs shifted strongly to the left (i.e. reversed), and more bicarbonate can be continuously reabsorbed from the serum. However, in the presence of acetazolamide, carbonic anhydrase is inhibited and carbonic acid levels build up. The inhibition of carbonic anhydrase in turn leads to a slowing of the reverse reaction and a decrease in the body's ability to reabsorb serum bicarbonate, resulting in urinary bicarbonate wasting. This leads to a decreased ability to exchange Na+ for H+ in the presence of acetazolamide (in proximal convulated tubules of kidney) resulting in a mild diuresis.[1] By contrast, the H+ that is also present in the lumen is reabsorbed via an alternative pathway along with Cl-; it then passes into the bloodstream, leading to hyperchloremic metabolic acidosis.[2] This effect can also be used for therapeutic correction of alkalosis seen in altitude sickness or other forms of respiratory alkalosis.
Uses
Acetazolamide is often used in the treatment of various diseases.
Glaucoma
Acetazolamide has been used for the treatment of suffers of glaucoma.[3] Acetazolamide decreases the formation of aqueous humor in the human eye, resulting in lower intraocular pressure.
Neurologic
In epilepsy, its main use is in absence seizures and myoclonic seizures.[4] It can be used in both episodic ataxia types 1 and 2 (although the mechanisms are presumed to be different between the two).
In catamential epilepsy, an increase in seizure frequency around menses, acetazolamide can be an adjunct to an anti-seizure medication regimen to aid in decreasing seizure frequency around menses.
Acetazolamide is also used to decrease the production of cerebrospinal fluid in idiopathic intracranial hypertension[5] and has shown efficacy in some forms of periodic paralysis.[6]
Marfan's syndrome
It's been demonstrated in drug trials to relieve symptoms associated with dural ectasia in individuals with Marfan's Syndrome.[7]
Central sleep apnea
Off-label uses include acetazolamide as a conjunction drug to merely assist patients with central sleep apnea by lowering blood pH and encourage respiration.[8]
Acute mountain sickness
To reduce the incidence of Acute Mountain Sickness acetazolamide is sometimes taken prophylactically, anywhere between 125 milligrams (mg) to 1000 mg per day,[9][10] starting a few days before going to higher altitudes. Such use is recommended for those ascending from sea level to 3000 meters (9800 feet) in one day, or for those ascending more than 600 meters (2000 feet) per day once above an altitude of 2500 meters (8200 feet).[11][12] Also, prophylactic use is recommended for those with a significant history of acute mountain sickness.
Acetazolamide forces the kidneys to excrete bicarbonate, the conjugate base of carbonic acid. By increasing the amount of bicarbonate excreted in the urine, the blood becomes more acidic.[12] Acidifying the blood stimulates ventilation, which increases the amount of oxygen in the blood.[13][14] At high altitudes, climbers hyperventilate in response to lower oxygen levels. The hyperventilation results in reduced carbon dioxide (an acid) and a respiratory alkalosis. The normal physiologic response to a respiratory alkalosis is for the kidneys to increase excretion of bicarbonate (a base) to compensate for the loss of carbon dioxide. This kidney response takes a few days, however acetazolamide in a sense accelerates this process by leading to a more rapid renal bicarbonate loss (metabolic acidosis).
Note that acetazolamide is not an immediate fix for acute mountain sickness; it speeds up part of the acclimatization process which in turn helps to relieve symptoms.[9][15] This may take up to a day or two, and requires waiting without any further rapid ascent. It is often advisable to descend if even mild acute mountain sickness is experienced. If serious sickness is encountered, descent to a lower elevation is considered to be mandatory unless other circumstances present greater danger.
Congestive Heart Failure
For diuresis in congestive heart failure, the starting dose is usually 250 to 375 mg once daily in the morning (five mg per kg). If, after an initial response, the patient fails to continue to lose edema fluid, do not increase the dose but allow for kidney recovery by skipping medication for a day.
Acetazolamide yields best diuretic results when given on alternate days, or for two days alternating with a day of rest.
Failures in therapy may be due to overdosage or too frequent dosage. The use of Acetazolamide does not eliminate the need for other therapy such as digitalis, bed rest, and salt restriction.
Drug-Induced Edema
Recommended dosage is 250 to 375 mg of Acetazolamide once a day for one or two days, alternating with a day of rest.
Note: The dosage recommendations for glaucoma and epilepsy differ considerably from those for congestive heart failure, since the first two conditions are not dependent upon carbonic anhydrase inhibition in the kidney which requires intermittent dosage if it is to recover from inhibitory effect of the therapeutic agent.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Side-effects
Common side effects of using this drug include numbness and tingling in the fingers and toes, and taste alterations (parageusia), especially for carbonated drinks. Some may also experience blurred vision but this usually disappears shortly after stopping the medication. Acetazolamide also increases the risk of developing calcium oxalate and calcium phosphate kidney stones. Everyone will experience more frequent urination as a result of using acetazolamide. One should drink more fluids than usual to prevent dehydration and headaches. Acetazolamide prolongs the effects of amphetamines and related drugs. Acetazolamide also causes metabolic acidosis.
Contraindications
Acetazolamide should not be taken by individuals if:
- They have sickle cell anemia
- They are allergic to sulfa medications
- They are allergic to any carbonic anhydrase inhibitor
- They have liver or kidney disease
- They have adrenal gland failure (i.e. Addison's disease)
- They are pregnant or are nursing mothers
Chemistry
Roblin, Richard O.; Clapp, James W. (1950). Journal of the American Chemical Society 72 (11): 4890. doi:10.1021/ja01167a011.
References
- ^ Lippincott's Illustrated Reviews: Pharmacology, 4th edition page 428
- ^ Renal and Electrolyte Disorders Schrier 1976: page 89
- ^ Kaur IP, Smitha R, Aggarwal D, Kapil M (November 2002). "Acetazolamide: future perspective in topical glaucoma therapeutics". Int J Pharm 248 (1–2): 1–14. doi:10.1016/S0378-5173(02)00438-6. PMID 12429455. http://linkinghub.elsevier.com/retrieve/pii/S0378517302004386.
- ^ "Treatment of Epilepsy | Comprehensive Epilepsy Center | NYU Medical Center, New York, NY". http://www.med.nyu.edu/cec/treatment/medications/side_effects/aceta.html. Retrieved 2008-12-19.
- ^ Celebisoy N, Gökçay F, Sirin H, Akyürekli O (November 2007). "Treatment of idiopathic intracranial hypertension: topiramate vs acetazolamide, an open-label study". Acta Neurol. Scand. 116 (5): 322–7. doi:10.1111/j.1600-0404.2007.00905.x. PMID 17922725. http://www3.interscience.wiley.com/resolve/openurl?genre=article&sid=nlm:pubmed&issn=0001-6314&date=2007&volume=116&issue=5&spage=322.
- ^ Ptáĉek LJ, Tawil R, Griggs RC, et al. (August 1994). "Sodium channel mutations in acetazolamide-responsive myotonia congenita, paramyotonia congenita, and hyperkalemic periodic paralysis". Neurology 44 (8): 1500–3. PMID 8058156.
- ^ Scoliosis Research Society (2006-11-27). "Dural Ectasia in the Marfan Spine: Symptoms and Treatment.also it's been used in high-altitude mountain sickness". SpineUniverse. http://www.spineuniverse.com/displayarticle.php/article922.html. Retrieved 2007-11-15.
- ^ White DP, Zwillich CW, Pickett CK, Douglas NJ, Findley LJ, Weil JV (October 1982). "Central sleep apnea. Improvement with acetazolamide therapy". Arch. Intern. Med. 142 (10): 1816–9. doi:10.1001/archinte.142.10.1816. PMID 6812522.
- ^ a b Cymerman, A; Rock, PB. Medical Problems in High Mountain Environments. A Handbook for Medical Officers. USARIEM-TN94-2. US Army Research Inst. of Environmental Medicine Thermal and Mountain Medicine Division Technical Report. http://archive.rubicon-foundation.org/7976. Retrieved 2010-09-06.
- ^ "FDA drug information, acetazolamide capsule, extended release". Barr Laboratories, Inc.. http://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=11119. Retrieved 2010-09-06.
- ^ Hackett, P.H. & Roach, R.C. (2001). "High-altitude illness". The New England Journal of Medicine 345 (2): 107–114. doi:10.1056/NEJM200107123450206. PMID 11450659.
- ^ a b Fulco, CS; Ditzler, D; Soares, R; Lammi, E; Muza, SR; Degroot, DW (2002). "Effect of Acetazolamide on Isolated Quadriceps Muscle Endurance Performance at Sea Level and During Acute Altitude Exposure". US Army Research Inst. of Environmental Medicine Thermal and Mountain Medicine Division Technical Report (USARIEM–TR–T02/9). http://archive.rubicon-foundation.org/7602. Retrieved 2008-09-30.
- ^ "Altitude.org". 2004. http://www.altitude.org. Retrieved 2009-06-05.
- ^ Leaf DE, Goldfarb DS (April 2007). "Mechanisms of action of acetazolamide in the prophylaxis and treatment of acute mountain sickness". J. Appl. Physiol. 102 (4): 1313–22. doi:10.1152/japplphysiol.01572.2005. PMID 17023566.
- ^ Muza, SR; Fulco, CS; Cymerman, A (2004). "Altitude Acclimatization Guide". US Army Research Inst. of Environmental Medicine Thermal and Mountain Medicine Division Technical Report (USARIEM–TN–04–05). http://archive.rubicon-foundation.org/7616. Retrieved 2009-03-05.
Anticonvulsants (N03) GABAA receptor agonist Clobazam • Clonazepam • Clorazepate • Diazepam# • Flutoprazepam • Lorazepam • Midazolam • Nimetazepam • Nitrazepam • TemazepamOther GABA agents Carbonic anhydrase inhibitor Channel blockers Primarily sodiumPrimarily calciumUnknown/ungroupedChannel openers PotassiumRetigabineIndirect GABA agents GABA transaminase inhibitor: Valproic acid# (Sodium valproate & Valproate semisodium) • Valpromide • Valnoctamide • Valproate pivoxil
GABA reuptake inhibitor: TiagabineUnknown/multiple/
unsortedPropionatesOphthalmologicals: antiglaucoma preparations and miotics (S01E) Sympathomimetics Parasympathomimetics muscarinicmuscarinic/nicotinicCarbonic anhydrase inhibitors/
(sulfonamides)Beta blocking agents Prostaglandin analogues (F2α) Other agents M: EYE
anat(g/a/p)/phys/devp/prot
noco/cong/tumr, epon
proc, drug(S1A/1E/1F/1L)
Antihypertensives: diuretics (C03) Sulfonamides
(except EA)CA inhibitors (at PT)AcetazolamideThiazide-likes (primarily DCT)Quinethazone • Clopamide • Chlortalidone • Mefruside • Clofenamide • Metolazone • Meticrane • Xipamide • Indapamide • Clorexolone • FenquizonePotassium-sparing (at CD) ESC blockersOsmotic diuretics (PT, DL) VAs (DCT and CD) vaptans: Conivaptan • Mozavaptan • Satavaptan • Tolvaptan
tetracyclines: DemeclocyclineOther Categories:- Carbonic anhydrase inhibitors
- Mountaineering and health
- Anticonvulsants
- Sulfonamides
- World Health Organization essential medicines
- Thiadiazoles
- Amides
Wikimedia Foundation. 2010.