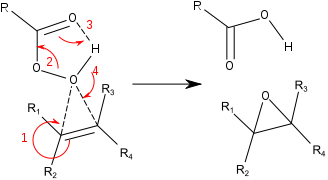- Epoxide
-
An epoxide is a cyclic ether with three ring atoms. This ring approximately defines an equilateral triangle, which makes it highly strained. The strained ring makes epoxides more reactive than other ethers. Simple epoxides are named from the parent compound ethylene oxide or oxirane, such as in chloromethyloxirane. As a functional group, epoxides feature the epoxy prefix, such as in the compound 1,2-epoxycycloheptane, which can also be called cycloheptene epoxide, or simply cycloheptene oxide.
 The chemical structure of the epoxide glycidol, a common chemical intermediate
The chemical structure of the epoxide glycidol, a common chemical intermediate
A polymer containing unreacted epoxide units is called a polyepoxide or an epoxy. Epoxy resins are used as adhesives and structural materials. Polymerization of an epoxide gives a polyether, for example ethylene oxide polymerizes to give polyethylene glycol, also known as polyethylene oxide.
Contents
Synthesis
The dominant epoxides industrially are ethylene oxide and propylene oxide, which are produced respectively on the scales of approximately 15 and 3 million tonnes.[1] The epoxidation of ethylene involves its catalytic reaction of oxygen according to the following stoichiometry:
- 7 H2C=CH2 + 6 O2 → 6 C2H4O + 2 CO2 + 2 H2O
The direct reaction of oxygen with alkenes is useful only for this epoxide. Other alkenes fail to react usefully, even propylene.
Olefin peroxidation
Most epoxides are generated by treating alkenes with peroxide-containing reagents, which donate a single oxygen atom. Typical peroxide reagents include hydrogen peroxide, peroxycarboxylic acids (generated in-situ or preformed), and alkyl hydroperoxides. In specialized applications, other peroxide-containing reagents are employed, such as dimethyldioxirane.
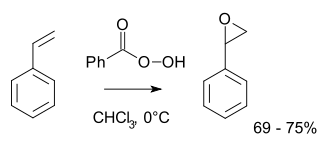
The largest scale application of this approach is the production of propylene oxide from propylene using either t-butyl hydroperoxide or ethylbenzene hydroperoxide.[2] More typically for laboratory operations, the Prilezhaev reaction is employed.[3][4] This approach involves the oxidation of the alkene with a peroxyacid such as m-CPBA. Illustrative is the epoxidation of styrene with perbenzoic acid to styrene oxide:[5]
The reaction proceeds via what is commonly known as the "Butterfly Mechanism."[6] The peroxide is viewed as an electrophile, and the alkene a nucleophile. The reaction is considered to be concerted (the numbers in the mechanism below are for simplification).
Hydroperoxides are also employed in catalytic enantioselective epoxidations, such as the Sharpless epoxidation and the Jacobsen epoxidation. In such cases, the oxygen is delivered from a metal oxide or peroxide. Together with the Shi epoxidation, these reactions are useful for the enantioselective synthesis of chiral epoxides. Oxaziridine reagents may also be used to generate epoxides from alkenes.
Intramolecular SN2 substitution
This method is a variant of the Williamson ether synthesis. In this case, an alkoxide ion displaces a chloride atom within the same molecule. The precursor compounds are called halohydrins. For example, with 2-chloropropanol:[7]
Approximately, half of the world's supply of propylene oxide arises via this route.[2] An intramolecular epoxide formation reaction is one of the key steps in the Darzens reaction.
In the Johnson-Corey-Chaykovsky reaction epoxides are generated from carbonyl groups and sulfonium ylides. In this reaction, a sulfonium is the leaving group instead of chloride.
Nucleophilic epoxidation
Electron-deficient olefins, such as enones and acryl derivatives can be epoxidized using nucleophilic oxygen compounds such as peroxides. The reaction is a two-step mechanism. First the oxygen performs a nucleophilic conjugate addition to give a stabilized carbanion. This carbanion then attacks the same oxygen atom, displacing a leaving group from it, to close the epoxide ring.
Asymmetric epoxidation
The carbon atoms of an epoxide are approximately sp3-hybridized, and thus may be stereogenic positions. Depending on the mechanism of the reaction and the geometry of the alkene starting material, cis and/or trans epoxide diastereomers may be formed. In addition, if there are other stereocenters present in the starting material, they can influence the stereochemistry of the epoxidation relative to them. This diastereoselectivity is a form of "substrate control" of the reaction. Finally, epoxidizing agents that possess stereogenic structures can influence the stereochemistry of the epoxide product (see for example the Sharpless epoxidation, Jacobsen epoxidation, and Juliá-Colonna Epoxidation). This enantioselectivity is a form of "reagent control" of the reaction.
Reactions
Typical epoxide reactions are listed below.
- Nucleophilic addition to an epoxide can be base or acid catalyzed.

- Under acidic conditions, the nucleophile attacks the carbon that will form the most stable carbocation, i.e. the most substituted carbon (similar to a halonium ion). Under basic conditions, the nucleophile attacks the least substituted carbon, in accordance with standard SN2 nucleophilic addition reaction process.
- Hydrolysis of an epoxide in presence of an acid catalyst generates a glycol. The hydrolysis process of epoxides can be considered to be the nucleophilic addition of water to the epoxide under acidic conditions.
- Reduction of an epoxide with lithium aluminium hydride and water generates an alcohol. This reduction process can be considered to be the nucleophilic addition of hydride (H-) to the epoxide under basic conditions.
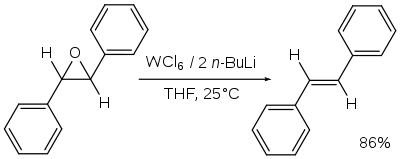
- Reduction with tungsten hexachloride and n-butyllithium generates the alkene. This reaction in effect is a de-epoxidation:[8]
- Reaction with the NH group in an amine. This covalent bond formation is utilised in epoxy glue with, e.g., Triethylenetetramine (TETA) as a hardener.
Perepoxides
Perepoxides are epoxides with an additional oxygen atom attached to the epoxide-oxygen. They are isoelectronic and isostructural with the cyclic sulfoxides derived from episulfides. Perepoxides are proposed intermediates in the photosensitized oxidation of alkenes, as occurs when drying oils (a component of some paints and varnishes) are exposed to air in light. Such intermediates arise from the addition of singlet oxygen to the double bond. Perepoxides rapidly rearrange to allylic hydroperoxides.[3]
See also
- Polyether
- Epoxide hydrolase
- Juliá-Colonna epoxidation
- Johnson-Corey-Chaykovsky reaction
References
- ^ Siegfried Rebsdat, Dieter Mayer "Ethylene Oxide" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005.doi:10.1002/14356007.a10_117 Article Online Posting Date: March 15, 2001.
- ^ a b Dietmar Kahlich, Uwe Wiechern, Jörg Lindner “Propylene Oxide” in Ullmann's Encyclopedia of Industrial Chemistry, 2002 by Wiley-VCH, Weinheim. doi:10.1002/14356007.a22_239Article Online Posting Date: June 15, 2000
- ^ a b March, Jerry. 1985. Advanced Organic Chemistry, Reactions, Mechanisms and Structure. 3rd ed. John Wiley & Sons. ISBN 0471854727.
- ^ Nikolaus Prileschajew (1909). "Oxydation ungesättigter Verbindungen mittels organischer Superoxyde". Berichte der deutschen chemischen Gesellschaft 42 (4): 4811–4815. doi:10.1002/cber.190904204100.
- ^ Harold Hibbert and Pauline Burt (1941), "Styrene Oxide", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv1p0494; Coll. Vol. 1: 494
- ^ Bartlett Rec. Chem. Prog 1950, 11 47.
- ^ Koppenhoefer, B.; Schurig, V. (1993), "(R)-Alkyloxiranes of High Enantiomeric Purity from (S)-2-Chloroalkanoic Acids via (S)-2-Chloro-1-Alkanols: (R)-Methyloxirane", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv8p0434; Coll. Vol. 8: 434
- ^ K. Barry Sharpless, Martha A. Umbreit, Marjorie T. Nieh, Thomas C. Flood (1972). "Lower valent tungsten halides. New class of reagents for deoxygenation of organic molecules". J. Am. Chem. Soc. 94 (18): 6538–6540. doi:10.1021/ja00773a045.
Functional groups Acetyl · Acetoxy · Acryloyl · Acyl · Alcohol · Aldehyde · Alkane · Alkene · Alkyne · Alkoxy group · Amide · Amine · Azo compound · Benzene derivative · Carboxylic acid · Cyanate · Disulfide · Ester · Ether · Epoxide · Haloalkane · Hydrazone · Hydroxyl · Imine · Isocyanate · Isonitrile · Isothiocyanate · Ketone · Methine · Nitrile · Nitro compound · Nitroso compound · Organophosphorus · Oxime · Peroxide · Phosphonous and Phosphonic acid · Pyridine derivative · Sulfone · Sulfonic acid · Sulfoxide · Thiocyanate · Thioester · Thioether · Thiol · Urea
See also Chemical classificationCategories:- Functional groups
- Epoxides
Wikimedia Foundation. 2010.