- Sulfide
-
For other uses, see Sulphide (disambiguation).
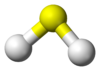
Sulfide 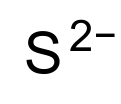
Identifiers CAS number 18496-25-8 
PubChem 29109 ChemSpider 27079 
ChEBI CHEBI:15138 Jmol-3D images Image 1 - [S--]
Properties Molecular formula S2− Molar mass 32.065 g mol-1 Exact mass 31.972070690 g mol-1 Related compounds Other anions Telluride Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references A sulfide is an anion of sulfur in its lowest oxidation state of 2-. Sulfide is also a slightly archaic term for thioethers, a common type of organosulfur compound that are well known for their bad odors.
Contents
Properties
The dianion S2− exists only in strongly alkaline aqueous solutions. Such solutions can form by dissolution of H2S or alkali metal salts such as Li2S, Na2S, and K2S in the presence of extra hydroxide. The ion S2− is exceptionally basic with a pKa > 14. It does not exist in appreciable concentrations even in highly alkaline water, being undetectable at pH < ~15 (8 M NaOH).[2]
Instead, sulfide combines with protons to form HS−, which is variously called hydrogen sulfide ion, hydrosulfide ion, sulfhydryl ion, or bisulfide ion. At still lower pH (<7), HS− converts to H2S, hydrogen sulfide.[3]
Sulfides are moderately strong reducing agents. They react with oxygen in the air in elevated temperatures to form higher-valence sulfur salts, such as sulfates and sulfur dioxide.
Metal derivatives
Aqueous solutions of transition metals cations react with sulfide sources (H2S, NaHS, Na2S) to precipitate solid sulfides. Such inorganic sulfides typically have very low solubility in water, and many are related to minerals with the same composition (see below). One famous example is the bright yellow species CdS or "cadmium yellow". The black tarnish formed on sterling silver is Ag2S. Such species are sometimes referred to as salts. In fact, the bonding in transition metal sulfides is highly covalent, which gives rise to their semiconductor properties, which in turn is related to the deep colors. Several have practical applications as pigments, in solar cells, and as catalysts.
Geology
Main article: sulfide mineralMany important metal ores are sulfides.[4] Significant examples include: argentite (silver sulfide), cinnabar (mercury), galena (lead sulfide), molybdenite (molybdenum sulfide), pentlandite (nickel sulfide]), realgar (arsenic sulfide), and stibnite (antimony), sphalerite (zinc sulfide), and pyrite (iron disulfide), and chalcopyrite (iron-copper sulfide).
Corrosion induced by sulfide
Dissolved free sulfides (H2S, HS− and S2−) are very aggressive species for the corrosion of many metals such as, e.g., steel, stainless steel, and copper. Sulfides present in aqueous solution are responsible for stress corrosion cracking (SCC) of steel, and is also known as sulfide stress cracking. Corrosion is a major concern in many industrial installations processing sulfides: sulfide ore mills, deep oil wells, pipeline transporting soured oil, Kraft paper factories. Microbially-induced corrosion (MIC) or biogenic sulfide corrosion are also caused by sulfate reducing bacteria producing sulfide.
Oxidation of sulfide can also form thiosulfate (S2O32−) an intermediate species responsible for severe problems of pitting corrosion of steel and stainless steel while the medium is also acidified by the production of sulfuric acid when oxidation is more advanced.
Organic chemistry
In organic chemistry, "sulfide" usually refers to the linkage C-S-C, although the term thioether is less ambiguous. For example, the thioether dimethyl sulfide is CH3-S-CH3. Polyphenylene sulfide (see below) has the empirical formula C6H4S. Occasionally, the term sulfide refers to molecules containing the -SH functional group. For example, methyl sulfide can mean CH3-SH. The preferred descriptor for such SH-containing compounds is thiol or mercaptan, i.e. methanethiol, or methyl mercaptan.
Disulfides
Confusion arises from the different meanings of the term "disulfide". Molybdenum disulfide (MoS2) consists of separated sulfide centers, in association with molybdenum in the formal 4+ oxidation state (Mo4+). Iron disulfide (pyrite, FeS2) on the other hand consists of S22−, or −S–S− dianion, in association with divalent iron in the formal 2+ oxidation state (ferrous ion: Fe2+). Dimethyldisulfide has the chemical binding CH3-S–S-CH3, whereas carbon disulfide has no S–S bond, being S=C=S (linear molecule analog to CO2). Most often in sulfur chemistry and in biochemistry, the disulfide term is commonly ascribed to the sulfur analogue of the peroxide −O–O− bond. The disulfide bond (−S–S−) plays a major role in the conformation of proteins and in the catalytic activity of enzymes.
Examples
Formula Melting point (°C) Boiling point (°C) CAS number H2S Hydrogen sulfide is a very toxic and corrosive gas characterised by a typical odour of "rotten egg". -85,7 -60,20 7783-06-4 CdS Cadmium sulfide can be used in photocells. 1750 1306-23-6 Calcium polysulfide ("lime sulfur") is a traditional fungicide in gardening. CS2 Carbon disulfide is sometimes used as a solvent in industrial chemistry. -111.6 46 75-15-0 PbS Lead sulfide is used in infra-red sensors. 1114 1314-87-0 MoS2 Molybdenum disulfide, the mineral molybdenite, is used as a catalyst to remove sulfur from fossil fuels; also as lubricant for high-temperature and high-pressure applications. 1317-33-5 Cl-CH2CH2-S-CH2CH2-Cl Sulfur mustard (mustard gas) is an organosulfide (thioether) that has been used as a chemical weapon in the First World War, the chloride on the molecule acts as a leaving group when in the presence of water and forms a thioether-alcohol and HCl. 13 - 14 217 505-60-2 Ag2S Silver sulfide is formed on silver electrical contacts operating in an atmosphere rich in hydrogen sulfide. 21548-73-2 Na2S Sodium sulfide is an important industrial chemical, used in manufacture of kraft paper, dyes, leather tanning, crude petroleum processing, treatment of heavy metal pollution, and others. 920 1180 1313-82-2 ZnS Zinc sulfide is used for lenses and other optical devices in the infrared part of the spectrum. Zinc sulfide doped with silver is used in alpha detectors while zinc sulfide with traces of copper has applications in photoluminescent strips for emergency lighting and luminous watch dials. 1185 1314-98-3 MeS Several metal sulfides are used as pigments in art, although their use has declined somewhat due to their toxicity. Sulfide pigments include cadmium, mercury, and arsenic. C6H4S Polyphenylene sulfide is a polymer commonly called "Sulfar". Its repeating units are bonded together by sulfide (thioether) linkages. 26125-40-6
25212-74-2SeS2 Selenium sulfide is an antifungal used in anti-dandruff preparations, such as Selsun Blue. The presence of the highly toxic selenium in healthcare and cosmetics products represents a general health and environmental concern. <100 7488-56-4 FeS2 The crystal lattice of pyrite is made of iron disulfide, in which iron is divalent and present as ferrous ion (Fe2+). 600 1317-66-4 Safety
Many metal sulfides are so insoluble in water that they are probably not very toxic. Some metal sulfides, when exposed to a strong mineral acid, including gastric acids, will release toxic hydrogen sulfide.
Organic sulfides are highly flammable. When a sulfide burns it produces sulfur dioxide (SO2) gas.
Hydrogen sulfide, some of its salts, and almost all organic sulfides have a strong and putrid stench; rotting biomass releases these.
See also
References
- ^ a b "sulfide(2-) (CHEBI:15138)". Chemical Entities of Biological Interest (ChEBI). UK: European Bioinformatics Institute. https://www.ebi.ac.uk/chebi/searchId.do?chebiId=15138.
- ^ Meyer B, Ward K, Koshlap K, Peter L "Second dissociation constant of hydrogen sulfide" Inorganic Chemistry 1983, volume 22, pp. 2345. doi:10.1021/ic00158a027.
- ^ Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the elements (2nd Edn.), Oxford:Butterworth-Heinemann. ISBN 0-7506-3365-4.
- ^ Vaughan, D. J.; Craig, J. R. “Mineral chemistry of metal sulfides" Cambridge University Press, Cambridge: 1978. ISBN 0521214890.
Categories:- Anions
- Corrosion
- Sulfides
- Functional groups
Wikimedia Foundation. 2010.