- Methcathinone
-
Methcathinone 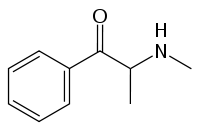
Systematic (IUPAC) name (RS)-2-(methylamino)-1-phenyl-propan-1-one Clinical data Pregnancy cat. ? Legal status Schedule I (US) Routes Vaporized, insufflated, injected, orally Pharmacokinetic data Excretion Urine Identifiers CAS number 5650-44-2  (R form)
(R form)
49656-78-2 (R form · HCl)
112117-24-5 (S form)
66514-93-0 (S form · HCl)ATC code None PubChem CID 1576 ChemSpider 1519 
Chemical data Formula C10H13NO Mol. mass 163.22 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Methcathinone (α-methylamino-propiophenone), is a psychoactive stimulant, sometimes used as a recreational drug and considered addictive.[1] It is usually snorted, but can be smoked, injected, or taken orally. Methcathinone is currently a DEA Schedule I controlled substance in the United States.
The C=O bond at the Rβ-position (directly right of the benzene ring) is slightly polar, and as a result the drug does not cross the lipid blood-brain barrier quite as well as amphetamine. Nevertheless, it is a potent CNS stimulant and dopamine reuptake inhibitor. Chronic high dosage use may result in acute mental confusion ranging from mild paranoia to psychosis. These symptoms typically disappear quickly if use is stopped.
Unlike methamphetamine, methcathinone is not legal under any circumstances in the US due to its classification as a Schedule I substance. Conversely, methamphetamine has certain approved medical uses such as treatment of morbid obesity, narcolepsy and ADHD.
Contents
History
Methcathinone was first synthesized in 1928 in the United States[2] and finally patented by Parke Davis in 1957[3]. It was used in the Soviet Union during the 1930s and 1940s as an anti-depressant. Since the 1960s, methcathinone has been used as a recreational drug in the (former) Soviet Union.
Circa 1994, the United States government recommended to the UN Secretary-General that methcathinone should be added to Schedule I of the Convention on Psychotropic Substances.[4]
Chemistry
Methcathinone is N-methylcathinone whereas cathine is N-demethylpseudoephedrine (SS), all of which are stimulant alkaloids found in the shrub Catha edulis (Khat). Stimulants such as meth/amphetamine, and other phenethylamines are also similar in structure.
Methcathinone possesses a chiral carbon atom, and therefore two enantiomers are possible. When it is made semi-synethically from pseudo/ephedrine as a starting material, then only a single enantiomer is produced. Given that the chiral centre is adjacent to the carbonyl group, the molecule will racemise in solution.
Methcathinone is "most commonly" made by the oxidation of ephedrine. Oxidation of ephedrine to methcathinone requires little chemistry experience, making it (relatively) easy to synthesize.[5] Potassium permanganate (KMnO4) is most commonly used as the oxidant.[6]
In clandestine laboratories, synthesizing methcathinone using either potassium permanganate or various chromates is considered undesirable because of the low yields and the high toxicity of these oxidants. A method that yields more methcathinone is oxidizing ephedrine with sodium hypochlorite.[7] If done in a proper laboratory using the proper procedures, however, potassium permanganate can be a high-yielding reactant.[6]
Methcathinone as free base is very unstable; it easily loses its ketone and converts back into an alcohol.[citation needed] Structurally, this occurs when the C=O bond at the Rβ-position is converted into a C-OH bond. In other words, basic methcathinone will turn into ephedrine, from which it was synthesized.
Effects
Methcathinone hydrochloride increases spontaneous rodent locomotor activity,[8] potentiates the release of dopamine from dopaminergic nerve terminals in the brain,[8] and causes appetite suppression.[citation needed] Users can easily forget to consume fluids leading to increased thirst and dehydration. The effects of methcathinone are similar to those of methamphetamine, initially deemed to be less intense by the inexperienced user, and often more euphoric.[citation needed] The effects have been compared to those of cocaine, since it commonly causes hypertension (elevated blood pressure) and tachycardia (elevated heart rate). Reported effects include:
- Feelings of euphoria
- Increased alertness
- Dilated pupils
- Slurred speech
- Increased heart rate
- Inability to stop talking
- Increased empathy and sense of communication
- Both decreased and increased sexual function and desire
- Loss of cognitive ability relating to the distinction of relative importance of matters
The effects of methcathinone usually last from four to six hours.
Use and pharmacology
Methcathinone has very strong affinities for the dopamine transporter and the noradrenaline transporter. Its affinity for the serotonin transporter is less than that of methamphetamine.[9]
Anecdotal reports have provided some information on patterns of methcathinone use. The most common route of administration is via nasal insufflation (snorting).[citation needed] Other routes of administration include per os, IV injection and smoking.
Methcathinone binges resemble amphetamine binges in that the user may not sleep or eat, and takes in little in the way of liquids. The methcathinone binge is followed by a "case of the Mondays" characterized by long periods of sleep, excess eating, long-lasting nosebleeds and, in some cases, depression.[citation needed]
Injecting this substance has recently been associated with symptoms similar to those seen in patients with Parkinson's Disease (Manganism) due to the compound manganese dioxide which is a byproduct of synthesis with permanganate.[10]
Street names
Street slang for methcathinone may be confused with methylmethcathinone (mephedrone) and terms may be used interchangeably and include terms such as MCat (pronounced "em-kat"), Cat, Meow, Stroof, Katestroof, Jeff, Bathtub Speed, Wannabe-Speed, Kitty, Meth's Cat, Meth's Kitten or Marzipan (from smell during synthesis).
In Europe, methcathinone is primarily known as Ephedrone.
In the United Kingdom, methcathinone may be known as M-cat (though this can also refer to the chemical 4-methylmethcathinone; known as mephedrone).
In Australia, methcathinone may be known as ketone or bk (beta ketone).
In South Africa it is known as Cat, Ietsies kayo, gram or line. There, it is also confused (via name alone, not presentation) with Khat (sometimes spelled Qat).
In Bulgaria it is known as PIKO.
In Poland it is known as Kot (cat)
Addiction
In preclinical studies, methcathinone hydrochloride produces an abuse potential similar to that of the amphetamines.[11]
Methcathinone can be highly psychologically addictive, and can produce a methamphetamine-like withdrawal, which is somewhat less in intensity than methamphetamine. It is highly unlikely for a methcathinone user to experience addiction on their first or even several subsequent administrations of the drug.
In drug discrimination studies, methcathinone hydrochloride evokes responses similar to those induced by both (+)-amphetamine sulfate and cocaine hydrochloride. When examined in particular pharmacological assays for psychomotor stimulant-like activity, both the d and l enantiomeric forms of methcathinone hydrochloride have been found to be pharmacologically active. In these assays, the l-form of methcathinone is more active than either d-methcathinone or (+)-amphetamine.
Clinical use
In the US, methcathinone is listed as a Schedule I drug, for which there is no clinical use. Research in the United States without a specific DEA permit is also prohibited. In the Netherlands, methylcathinone is listed as a Level I substance of the Opium Law, for which there is no clinical use. In the UK, methcathinone is listed as a Class B drug,[12] with no clinical uses.
See also
- Substituted cathinone
References
- ^ Calkins RF, Aktan GB, Hussain KL (1995). "Methcathinone: the next illicit stimulant epidemic?". Journal of psychoactive drugs 27 (3): 277–85. PMID 8594170.
- ^ Hyde JF, Browning E, Adams R (1928). "Synthetic Homologs of d,l-Ephedrine". Journal of the American Chemical Society 50 (8): 2287–2292. doi:10.1021/ja01395a032.
- ^ US Patent 2802865 -ETHYLAMINOPROPIOPHENONE COMPOUNDS
- ^ Erowid
- ^ http://www.scribd.com/doc/35411/The-Clandestine-Chemists-Notebook The Clandestine Chemists Notebook
- ^ a b Methcathinone HCl FAQ v2.2
- ^ http://designer-drugs.com/pte/12.162.180.114/dcd/chemistry/methcathinone.txt
- ^ a b Glennon RA, Yousif M, Naiman N, Kalix P (1987). "Methcathinone: a new and potent amphetamine-like agent". Pharmacol. Biochem. Behav. 26 (3): 547–51. doi:10.1016/0091-3057(87)90164-X. PMID 3575369.
- ^ Rothman, B. R. et al. (June 2003). "In Vitro Characterization of Ephedrine-Related Stereoisomers at Biogenic Amine Transporters and the Receptorome Reveals Selective Actions as Norepinephrine Transporter Substrates". The Journal of Pharmacology and Experimental Therapeutics 307 (1): 138–45. doi:10.1124/jpet.103.053975. PMID 12954796.
- ^ De Bie RM, Gladstone RM, Strafella AP, Ko JH, Lang AE (Jun 2007). "Manganese-induced Parkinsonism associated with methcathinone (Ephedrone) abuse". Archives of Neurology 64 (6): 886–9. doi:10.1001/archneur.64.6.886. PMID 17562938.
- ^ Kaminski BJ, Griffiths RR (April 1994). Intravenous self-injection of methcathinone in the baboon. 47. pp. 981–3. PMID 8029273.
- ^ "The Misuse of Drugs Act 1971 (Modification) Order 1998 (SI 1998 No. 750)". Statutory Instrument. Ministry of Justice. 1998-03-18. http://www.statutelaw.gov.uk/content.aspx?ActiveTextDocId=2810429. Retrieved 2008-07-06.
- International Drug Scheduling; Convention on Psychotropic Substances; Certain Stimulant/Hallucinogenic Drugs and Certain Nonbarbiturate Sedative Drugs, Food and Drug Administration, June 20, 1994.
External links
Stimulants (N06B) Adamantanes Adaphenoxate • Adapromine • Amantadine • Bromantane • Chlodantane • Gludantane • Memantine • Midantane
Adenosine antagonists 8-Chlorotheophylline • 8-Cyclopentyltheophylline • 8-Phenyltheophylline • Aminophylline • Caffeine • CGS-15943 • Dimethazan • Paraxanthine • SCH-58261 • Theobromine • TheophyllineAlkylamines Arylcyclohexylamines Benocyclidine • Dieticyclidine • Esketamine • Eticyclidine • Gacyclidine • Ketamine • Phencyclamine • Phencyclidine • Rolicyclidine • Tenocyclidine • Tiletamine
Benzazepines 6-Br-APB • SKF-77434 • SKF-81297 • SKF-82958
Cholinergics A-84543 • A-366,833 • ABT-202 • ABT-418 • AR-R17779 • Altinicline • Anabasine • Arecoline • Cotinine • Cytisine • Dianicline • Epibatidine • Epiboxidine • GTS-21 • Ispronicline • Nicotine • PHA-543,613 • PNU-120,596 • PNU-282,987 • Pozanicline • Rivanicline • Sazetidine A • SIB-1553A • SSR-180,711 • TC-1698 • TC-1827 • TC-2216 • TC-5619 • Tebanicline • UB-165 • Varenicline • WAY-317,538
Convulsants Anatoxin-a • Bicuculline • DMCM • Flurothyl • Gabazine • Pentetrazol • Picrotoxin • Strychnine • Thujone
Eugeroics Adrafinil • Armodafinil • CRL-40941 • Modafinil
Oxazolines 4-Methylaminorex • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone
Phenethylamines 1-(4-Methylphenyl)-2-aminobutane • 1-Phenyl-2-(piperidin-1-yl)pentan-3-one • 1-Methylamino-1-(3,4-methylenedioxyphenyl)propane • 2-Fluoroamphetamine • 2-Fluoromethamphetamine • 2-OH-PEA • 2-Phenyl-3-aminobutane • 2-Phenyl-3-methylaminobutane • 2,3-MDA • 3-Fluoroamphetamine • 3-Fluoroethamphetamine • 3-Fluoromethcathinone • 3-Methoxyamphetamine • 3-Methylamphetamine • 3,4-DMMC • 4-BMC • 4-Ethylamphetamine • 4-FA • 4-FMA • 4-MA • 4-MMA • 4-MTA • 6-FNE • Alfetamine • α-Ethylphenethylamine • Amfecloral • Amfepentorex • Amfepramone • Amidephrine • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • Arbutamine • β-Methylphenethylamine • β-Phenylmethamphetamine • Benfluorex • Benzedrone • Benzphetamine • BDB (J) • BOH (Hydroxy-J) • BPAP • Buphedrone • Bupropion (Amfebutamone) • Butylone • Cathine • Cathinone • Chlorphentermine • Cinnamedrine • Clenbuterol • Clobenzorex • Cloforex • Clortermine • D-Deprenyl • Denopamine • Dimethoxyamphetamine • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, Metamfepramone) • Dobutamine • DOPA (Dextrodopa, Levodopa) • Dopamine • Dopexamine • Droxidopa • EBDB (Ethyl-J) • Ephedrine • Epinephrine (Adrenaline) • Epinine (Deoxyepinephrine) • Etafedrine • Ethcathinone (Ethylpropion) • Ethylamphetamine (Etilamfetamine) • Ethylnorepinephrine (Butanefrine) • Ethylone • Etilefrine • Famprofazone • Fenbutrazate • Fencamine • Fenethylline • Fenfluramine (Dexfenfluramine) • Fenmetramide • Fenproporex • Flephedrone • Fludorex • Furfenorex • Gepefrine • HMMA • Hordenine • Ibopamine • IMP • Indanylamphetamine • Isoetarine • Isoethcathinone • Isoprenaline (Isoproterenol) • L-Deprenyl (Selegiline) • Lefetamine • Lisdexamfetamine • Lophophine (Homomyristicylamine) • Manifaxine • MBDB (Methyl-J; "Eden") • MDA (Tenamfetamine) • MDBU • MDEA ("Eve") • MDMA ("Ecstasy", "Adam") • MDMPEA (Homarylamine) • MDOH • MDPR • MDPEA (Homopiperonylamine) • Mefenorex • Mephedrone • Mephentermine • Metanephrine • Metaraminol • Methamphetamine (Desoxyephedrine, Methedrine; Dextromethamphetamine, Levomethamphetamine) • Methoxamine • Methoxyphenamine • MMA • Methcathinone (Methylpropion) • Methedrone • Methoxyphenamine • Methylone • MMDA • MMDMA • MMMA • Morazone • N-Benzyl-1-phenethylamine • N,N-Dimethylphenethylamine • Naphthylamphetamine • Nisoxetine • Norepinephrine (Noradrenaline) • Norfenefrine • Norfenfluramine • Normetanephrine • Octopamine • Orciprenaline • Ortetamine • Oxilofrine • Paredrine (Norpholedrine, Oxamphetamine, Mycadrine) • PBA • PCA • PHA • Pargyline • Pentorex (Phenpentermine) • Pentylone • Phendimetrazine • Phenmetrazine • Phenpromethamine • Phentermine • Phenylalanine • Phenylephrine (Neosynephrine) • Phenylpropanolamine • Pholedrine • PIA • PMA • PMEA • PMMA • PPAP • Prenylamine • Propylamphetamine • Pseudoephedrine • Radafaxine • Ropinirole • Salbutamol (Albuterol; Levosalbutamol) • Sibutramine • Synephrine (Oxedrine) • Theodrenaline • Tiflorex (Flutiorex) • Tranylcypromine • Tyramine • Tyrosine • Xamoterol • Xylopropamine • Zylofuramine
Piperazines Piperidines 1-Benzyl-4-(2-(diphenylmethoxy)ethyl)piperidine • 1-(3,4-Dichlorophenyl)-1-(piperidin-2-yl)butane • 2-Benzylpiperidine • 2-Methyl-3-phenylpiperidine • 3,4-Dichloromethylphenidate • 4-Benzylpiperidine • 4-Methylmethylphenidate • Desoxypipradrol • Difemetorex • Diphenylpyraline • Ethylphenidate • Methylnaphthidate • Methylphenidate (Dexmethylphenidate) • N-Methyl-3β-propyl-4β-(4-chlorophenyl)piperidine • Nocaine • Phacetoperane • Pipradrol • SCH-5472
Pyrrolidines 2-Diphenylmethylpyrrolidine • α-PPP • α-PBP • α-PVP • Diphenylprolinol • MDPPP • MDPBP • MDPV • MPBP • MPHP • MPPP • MOPPP • Naphyrone • PEP • Prolintane • Pyrovalerone
Tropanes 3-CPMT • 3'-Chloro-3α-(diphenylmethoxy)tropane • 3-Pseudotropyl-4-fluorobenzoate • 4'-Fluorococaine • AHN-1055 • Altropane (IACFT) • Brasofensine • CFT (WIN 35,428) • β-CIT (RTI-55) • Cocaethylene • Cocaine • Dichloropane (RTI-111) • Difluoropine • FE-β-CPPIT • FP-β-CPPIT • Ioflupane (123I) • Norcocaine • PIT • PTT • RTI-31 • RTI-32 • RTI-51 • RTI-105 • RTI-112 • RTI-113 • RTI-117 • RTI-120 • RTI-121 (IPCIT) • RTI-126 • RTI-150 • RTI-154 • RTI-171 • RTI-177 • RTI-183 • RTI-193 • RTI-194 • RTI-199 • RTI-202 • RTI-204 • RTI-229 • RTI-241 • RTI-336 • RTI-354 • RTI-371 • RTI-386 • Salicylmethylecgonine • Tesofensine • Troparil (β-CPT, WIN 35,065-2) • Tropoxane • WF-23 • WF-33 • WF-60
Others 1-(Thiophen-2-yl)-2-aminopropane • 2-Amino-1,2-dihydronaphthalene • 2-Aminoindane • 2-Aminotetralin • 2-MDP • 2-Phenylcyclohexylamine • 2-Phenyl-3,6-dimethylmorpholine • 3-Benzhydrylmorpholine • 3,3-Diphenylcyclobutanamine • 5-(2-Aminopropyl)indole • 5-Iodo-2-aminoindane • AL-1095 • Amfonelic acid • Amineptine • Amiphenazole • Atipamezole • Atomoxetine (Tomoxetine) • Bemegride • Benzydamine • BTQ • BTS 74,398 • Carphedon • Ciclazindol • Cilobamine • Clofenciclan • Cropropamide • Crotetamide • Cypenamine • D-161 • Diclofensine • Dimethocaine • Efaroxan • Etamivan • EXP-561 • Fencamfamine • Fenpentadiol • Feprosidnine • G-130 • Gamfexine • Gilutensin • GSK1360707F • GYKI-52895 • Hexacyclonate • Idazoxan • Indanorex • Indatraline • JNJ-7925476 • JZ-IV-10 • Lazabemide • Leptacline • Levopropylhexedrine • Lomevactone • LR-5182 • Mazindol • Meclofenoxate • Medifoxamine • Mefexamide • Mesocarb • Methastyridone • Methiopropamine • N-Methyl-3-phenylnorbornan-2-amine • Nefopam • Nikethamide • Nomifensine • O-2172 • Oxaprotiline • Phthalimidopropiophenone • PNU-99,194 • Propylhexedrine • PRC200-SS • Rasagiline • Rauwolscine • Rubidium chloride • Setazindol • Tametraline • Tandamine • Trazium • UH-232 • Yohimbine
See also Sympathomimetic amines Adrenergics Receptor ligands Agonists: 5-FNE • 6-FNE • Amidephrine • Anisodamine • Anisodine • Cirazoline • Dipivefrine • Dopamine • Ephedrine • Epinephrine (Adrenaline) • Etilefrine • Ethylnorepinephrine • Indanidine • Levonordefrin • Metaraminol • Methoxamine • Methyldopa • Midodrine • Naphazoline • Norepinephrine (Noradrenaline) • Octopamine • Oxymetazoline • Phenylephrine • Phenylpropanolamine • Pseudoephedrine • Synephrine • Tetrahydrozoline
Antagonists: Abanoquil • Adimolol • Ajmalicine • Alfuzosin • Amosulalol • Arotinolol • Atiprosin • Benoxathian • Buflomedil • Bunazosin • Carvedilol • CI-926 • Corynanthine • Dapiprazole • DL-017 • Domesticine • Doxazosin • Eugenodilol • Fenspiride • GYKI-12,743 • GYKI-16,084 • Indoramin • Ketanserin • L-765,314 • Labetalol • Mephendioxan • Metazosin • Monatepil • Moxisylyte (Thymoxamine) • Naftopidil • Nantenine • Neldazosin • Nicergoline • Niguldipine • Pelanserin • Phendioxan • Phenoxybenzamine • Phentolamine • Piperoxan • Prazosin • Quinazosin • Ritanserin • RS-97,078 • SGB-1,534 • Silodosin • SL-89.0591 • Spiperone • Talipexole • Tamsulosin • Terazosin • Tibalosin • Tiodazosin • Tipentosin • Tolazoline • Trimazosin • Upidosin • Urapidil • Zolertine
* Note that many TCAs, TeCAs, antipsychotics, ergolines, and some piperazines like buspirone, trazodone, nefazodone, etoperidone, and mepiprazole all antagonize α1-adrenergic receptors as well, which contributes to their side effects such as orthostatic hypotension.Agonists: (R)-3-Nitrobiphenyline • 4-NEMD • 6-FNE • Amitraz • Apraclonidine • Brimonidine • Cannabivarin • Clonidine • Detomidine • Dexmedetomidine • Dihydroergotamine • Dipivefrine • Dopamine • Ephedrine • Ergotamine • Epinephrine (Adrenaline) • Esproquin • Etilefrine • Ethylnorepinephrine • Guanabenz • Guanfacine • Guanoxabenz • Levonordefrin • Lofexidine • Medetomidine • Methyldopa • Mivazerol • Naphazoline • Norepinephrine (Noradrenaline) • Phenylpropanolamine • Piperoxan • Pseudoephedrine • Rilmenidine • Romifidine • Talipexole • Tetrahydrozoline • Tizanidine • Tolonidine • Urapidil • Xylazine • Xylometazoline
Antagonists: 1-PP • Adimolol • Aptazapine • Atipamezole • BRL-44408 • Buflomedil • Cirazoline • Efaroxan • Esmirtazapine • Fenmetozole • Fluparoxan • GYKI-12,743 • GYKI-16,084 • Idazoxan • Mianserin • Mirtazapine • MK-912 • NAN-190 • Olanzapine • Phentolamine • Phenoxybenzamine • Piperoxan • Piribedil • Rauwolscine • Rotigotine • SB-269,970 • Setiptiline • Spiroxatrine • Sunepitron • Tolazoline • Yohimbine
* Note that many atypical antipsychotics and azapirones like buspirone and gepirone (via metabolite 1-PP) antagonize α2-adrenergic receptors as well.βAgonists: 2-FNE • 5-FNE • Amibegron • Arbutamine • Arformoterol • Arotinolol • BAAM • Bambuterol • Befunolol • Bitolterol • Broxaterol • Buphenine • Carbuterol • Cimaterol • Clenbuterol • Denopamine • Deterenol • Dipivefrine • Dobutamine • Dopamine • Dopexamine • Ephedrine • Epinephrine (Adrenaline) • Etafedrine • Etilefrine • Ethylnorepinephrine • Fenoterol • Formoterol • Hexoprenaline • Higenamine • Indacaterol • Isoetarine • Isoprenaline (Isoproterenol) • Isoxsuprine • Labetalol • Levonordefrin • Levosalbutamol • Mabuterol • Methoxyphenamine • Methyldopa • Norepinephrine (Noradrenaline) • Orciprenaline • Oxyfedrine • Phenylpropanolamine • Pirbuterol • Prenalterol • Ractopamine • Procaterol • Pseudoephedrine • Reproterol • Rimiterol • Ritodrine • Salbutamol (Albuterol) • Salmeterol • Solabegron • Terbutaline • Tretoquinol • Tulobuterol • Xamoterol • Zilpaterol • Zinterol
Antagonists: Acebutolol • Adaprolol • Adimolol • Afurolol • Alprenolol • Alprenoxime • Amosulalol • Ancarolol • Arnolol • Arotinolol • Atenolol • Befunolol • Betaxolol • Bevantolol • Bisoprolol • Bopindolol • Bormetolol • Bornaprolol • Brefonalol • Bucindolol • Bucumolol • Bufetolol • Buftiralol • Bufuralol • Bunitrolol • Bunolol • Bupranolol • Burocrolol • Butaxamine • Butidrine • Butofilolol • Capsinolol • Carazolol • Carpindolol • Carteolol • Carvedilol • Celiprolol • Cetamolol • Cicloprolol • Cinamolol • Cloranolol • Cyanopindolol • Dalbraminol • Dexpropranolol • Diacetolol • Dichloroisoprenaline • Dihydroalprenolol • Dilevalol • Diprafenone • Draquinolol • Dropranolol • Ecastolol • Epanolol • Ericolol • Ersentilide • Esatenolol • Esmolol • Esprolol • Eugenodilol • Exaprolol • Falintolol • Flestolol • Flusoxolol • Hydroxycarteolol • Hydroxytertatolol • ICI-118,551 • Idropranolol • Indenolol • Indopanolol • Iodocyanopindolol • Iprocrolol • Isoxaprolol • Isamoltane • Labetalol • Landiolol • Levobetaxolol • Levobunolol • Levocicloprolol • Levomoprolol • Medroxalol • Mepindolol • Metalol • Metipranolol • Metoprolol • Moprolol • Nadolol • Nadoxolol • Nafetolol • Nebivolol • Neraminol • Nifenalol • Nipradilol • Oberadilol • Oxprenolol • Pacrinolol • Pafenolol • Pamatolol • Pargolol • Parodilol • Penbutolol • Penirolol • PhQA-33 • Pindolol • Pirepolol • Practolol • Primidolol • Procinolol • Pronethalol • Propafenone • Propranolol • Ridazolol • Ronactolol • Soquinolol • Sotalol • Spirendolol • SR 59230A • Sulfinalol • TA-2005 • Talinolol • Tazolol • Teoprolol • Tertatolol • Terthianolol • Tienoxolol • Tilisolol • Timolol • Tiprenolol • Tolamolol • Toliprolol • Tribendilol • Trigevolol • Xibenolol • XipranololReuptake inhibitors Selective norepinephrine reuptake inhibitors: Amedalin • Atomoxetine (Tomoxetine) • Ciclazindol • Daledalin • Esreboxetine • Lortalamine • Mazindol • Nisoxetine • Reboxetine • Talopram • Talsupram • Tandamine • Viloxazine; Norepinephrine-dopamine reuptake inhibitors: Amineptine • Bupropion (Amfebutamone) • Fencamine • Fencamfamine • Lefetamine • Levophacetoperane • LR-5182 • Manifaxine • Methylphenidate • Nomifensine • O-2172 • Radafaxine; Serotonin-norepinephrine reuptake inhibitors: Bicifadine • Desvenlafaxine • Duloxetine • Eclanamine • Levomilnacipran • Milnacipran • Sibutramine • Venlafaxine; Serotonin-norepinephrine-dopamine reuptake inhibitors: Brasofensine • Diclofensine • DOV-102,677 • DOV-21,947 • DOV-216,303 • JNJ-7925476 • JZ-IV-10 • Methylnaphthidate • Naphyrone • NS-2359 • PRC200-SS • SEP-225,289 • SEP-227,162 • Tesofensine; Tricyclic antidepressants: Amitriptyline • Butriptyline • Cianopramine • Clomipramine • Desipramine • Dosulepin • Doxepin • Imipramine • Lofepramine • melitracen • Nortriptyline • Protriptyline • Trimipramine; Tetracyclic antidepressants: Amoxapine • Maprotiline • Mianserin • Oxaprotiline • Setiptiline; Others: Cocaine • CP-39,332 • EXP-561 • Fezolamine • Ginkgo biloba • Indeloxazine • Nefazodone • Nefopam • Pridefrine • Tapentadol • Teniloxazine • Tramadol • ZiprasidoneEnzyme inhibitors 3,4-DihydroxystyreneDBHCGS-19281A • SKF-64139 • SKF-7698Nonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima; MAO-B selective: D-Deprenyl • Selegiline (L-Deprenyl) • Ladostigil • Lazabemide • Milacemide • Mofegiline • Pargyline • Rasagiline • Safinamide
* Note that MAO-B inhibitors also influence norepinephrine/epinephrine levels since they inhibit the breakdown of their precursor dopamine.COMTOthers Ferrous Iron (Fe2+) • S-Adenosyl-L-Methionine • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal Phosphate) • Vitamin B9 (Folic acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersActivity enhancers: BPAP • PPAP; Release blockers: Bethanidine • Bretylium • Guanadrel • Guanazodine • Guanclofine • Guanethidine • Guanoxan; Toxins: Oxidopamine (6-Hydroxydopamine)List of adrenergic drugs Dopaminergics Reuptake inhibitors PlasmalemmalDAT inhibitorsPiperazines: DBL-583 • GBR-12,935 • Nefazodone • Vanoxerine; Piperidines: BTCP • Desoxypipradrol • Dextromethylphenidate • Difemetorex • Ethylphenidate • Methylnaphthidate • Methylphenidate • Phencyclidine • Pipradrol; Pyrrolidines: Diphenylprolinol • Methylenedioxypyrovalerone (MDPV) • Naphyrone • Prolintane • Pyrovalerone; Tropanes: β-CPPIT • Altropane • Brasofensine • CFT • Cocaine • Dichloropane • Difluoropine • FE-β-CPPIT • FP-β-CPPIT • Ioflupane (123I) • Iometopane • RTI-112 • RTI-113 • RTI-121 • RTI-126 • RTI-150 • RTI-177 • RTI-229 • RTI-336 • Tenocyclidine • Tesofensine • Troparil • Tropoxane • WF-11 • WF-23 • WF-31 • WF-33; Others: Adrafinil • Armodafinil • Amfonelic acid • Amineptine • Benzatropine (Benztropine) • Bromantane • BTQ • BTS-74,398 • Bupropion (Amfebutamone) • Ciclazindol • Diclofensine • Dimethocaine • Diphenylpyraline • Dizocilpine • DOV-102,677 • DOV-21,947 • DOV-216,303 • Etybenzatropine (Ethylbenztropine) • EXP-561 • Fencamine • Fencamfamine • Fezolamine • GYKI-52,895 • Indatraline • Ketamine • Lefetamine • Levophacetoperane • LR-5182 • Manifaxine • Mazindol • Medifoxamine • Mesocarb • Modafinil • Nefopam • Nomifensine • NS-2359 • O-2172 • Pridefrine • Propylamphetamine • Radafaxine • SEP-225,289 • SEP-227,162 • Sertraline • Sibutramine • Tametraline • TripelennamineVMAT inhibitorsReleasing agents Morpholines: Fenbutrazate • Morazone • Phendimetrazine • Phenmetrazine; Oxazolines: 4-Methylaminorex (4-MAR, 4-MAX) • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone; Phenethylamines (also amphetamines, cathinones, phentermines, etc): 2-Hydroxyphenethylamine (2-OH-PEA) • 4-CAB • 4-Methylamphetamine (4-MA) • 4-Methylmethamphetamine (4-MMA) • Alfetamine • Amfecloral • Amfepentorex • Amfepramone • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • β-Methylphenethylamine (β-Me-PEA) • Benzodioxolylbutanamine (BDB) • Benzodioxolylhydroxybutanamine (BOH) • Benzphetamine • Buphedrone • Butylone • Cathine • Cathinone • Clobenzorex • Clortermine • D-Deprenyl • Dimethoxyamphetamine (DMA) • Dimethoxymethamphetamine (DMMA) • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, metamfepramone) • Ethcathinone (Ethylpropion) • Ethylamphetamine • Ethylbenzodioxolylbutanamine (EBDB) • Ethylone • Famprofazone • Fenethylline • Fenproporex • Flephedrone • Fludorex • Furfenorex • Hordenine • Lophophine (Homomyristicylamine) • Mefenorex • Mephedrone • Methamphetamine (Desoxyephedrine, Methedrine; Dextromethamphetamine, Levomethamphetamine) • Methcathinone (Methylpropion) • Methedrone • Methoxymethylenedioxyamphetamine (MMDA) • Methoxymethylenedioxymethamphetamine (MMDMA) • Methylbenzodioxolylbutanamine (MBDB) • Methylenedioxyamphetamine (MDA, tenamfetamine) • Methylenedioxyethylamphetamine (MDEA) • Methylenedioxyhydroxyamphetamine (MDOH) • Methylenedioxymethamphetamine (MDMA) • Methylenedioxymethylphenethylamine (MDMPEA, homarylamine) • Methylenedioxyphenethylamine (MDPEA, homopiperonylamine) • Methylone • Ortetamine • Parabromoamphetamine (PBA) • Parachloroamphetamine (PCA) • Parafluoroamphetamine (PFA) • Parafluoromethamphetamine (PFMA) • Parahydroxyamphetamine (PHA) • Paraiodoamphetamine (PIA) • Paredrine (Norpholedrine, Oxamphetamine) • Phenethylamine (PEA) • Pholedrine • Phenpromethamine • Prenylamine • Propylamphetamine • Tiflorex (Flutiorex) • Tyramine (TRA) • Xylopropamine • Zylofuramine; Piperazines: 2,5-Dimethoxy-4-bromobenzylpiperazine (2C-B-BZP) • Benzylpiperazine (BZP) • Methoxyphenylpiperazine (MeOPP, paraperazine) • Methylbenzylpiperazine (MBZP) • Methylenedioxybenzylpiperazine (MDBZP, piperonylpiperazine); Others: 2-Amino-1,2-dihydronaphthalene (2-ADN) • 2-Aminoindane (2-AI) • 2-Aminotetralin (2-AT) • 4-Benzylpiperidine (4-BP) • 5-IAI • Clofenciclan • Cyclopentamine • Cypenamine • Cyprodenate • Feprosidnine • Gilutensin • Heptaminol • Hexacyclonate • Indanylaminopropane (IAP) • Indanorex • Isometheptene • Methylhexanamine • Naphthylaminopropane (NAP) • Octodrine • Phthalimidopropiophenone • Propylhexedrine (Levopropylhexedrine) • Tuaminoheptane (Tuamine)Enzyme inhibitors PAH inhibitors3,4-DihydroxystyreneTH inhibitorsNonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima; MAO-B selective: D-Deprenyl • L-Deprenyl (Selegiline) • Ladostigil • Lazabemide • Milacemide • Pargyline • Rasagiline • SafinamideDBH inhibitorsOthers L-Phenylalanine → L-Tyrosine → L-DOPA (Levodopa)Ferrous iron (Fe2+) • Tetrahydrobiopterin • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal phosphate) • Vitamin B9 (Folic acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersActivity enhancers: Benzofuranylpropylaminopentane (BPAP) • Phenylpropylaminopentane (PPAP); Toxins: Oxidopamine (6-Hydroxydopamine)List of dopaminergic drugs Phenethylamines Phenethylamines Psychedelics: 2C-B • 2C-B-FLY • 2C-C • 2C-D • 2C-E • 2C-F • 2C-G • 2C-I • 2C-N • 2C-P • 2C-SE • 2C-T • 2C-T-2 • 2C-T-4 • 2C-T-7 • 2C-T-8 • 2C-T-9 • 2C-T-13 • 2C-T-15 • 2C-T-17 • 2C-T-21 • 2C-TFM • 2C-YN • Allylescaline • DESOXY • Escaline • Isoproscaline • Jimscaline • Macromerine • MEPEA • Mescaline • Metaescaline • Methallylescaline • Proscaline • Psi-2C-T-4 • TCB-2
Stimulants: 2-OH-PEA • β-Me-PEA • Hordenine • N-Me-PEA • Phenethylamine (PEA)
Entactogens: Lophophine • MDPEA • MDMPEA
Others: BOH • DMPEAAmphetamines
PhenylisopropylaminesPsychedelics: 3C-BZ • 3C-E • 3C-P • Aleph • Beatrice • Bromo-DragonFLY • D-Deprenyl • DMA • DMCPA • DMMDA • DOB • DOC • DOEF • DOET • DOI • DOM • DON • DOPR • DOTFM • Ganesha • MMDA • MMDA-2 • Psi-DOM • TMA • TeMA
Stimulants: 4-MA • 4-MMA • 4-MTA • 5-IT • Alfetamine • Amfecloral • Amfepentorex • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • Benfluorex • Benzphetamine • Cathine • Clobenzorex • Dimethylamphetamine • Ephedrine (EPH) • Ethylamphetamine • Fencamfamine • Fencamine • Fenethylline • Fenfluramine (Dexfenfluramine) • Fenproporex • Fludorex • Furfenorex • Isopropylamphetamine • Lefetamine • Mefenorex • Methamphetamine (Dextromethamphetamine, Levomethamphetamine) • Methoxyphenamine • MMA • Norfenfluramine • Oxilofrine • Ortetamine • PBA • PCA • Phenpromethamine • PFA • PFMA • PIA • PMA • PMEA • PMMA • Phenylpropanolamine (PPA) • Prenylamine • Propylamphetamine • Pseudoephedrine (PSE) • Sibutramine • Tiflorex (Flutiorex) • Tranylcypromine • Xylopropamine • Zylofuramine
Entactogens: 5-APDB • 6-APB • 6-APDB • EDA • IAP • MDA • MDEA • MDHMA (FLEA) • MDMA ("Ecstasy") • MDOH • MMDMA • NAP • TAP
Others: Amiflamine • DFMDA • D-Deprenyl • L-Deprenyl (Selegiline)Phentermines Stimulants: Chlorphentermine • Cloforex • Clortermine • Etolorex • Mephentermine • Pentorex (Phenpentermine) • Phentermine
Entactogens: MDPH • MDMPHCathinones Stimulants: Amfepramone • Brephedrone • Buphedrone • Bupropion (Amfebutamone) • Cathinone (Propion) • Dimethylcathinone (Dimethylpropion, Metamfepramone) • Ethcathinone (Ethylpropion) • Flephedrone • Methcathinone (Methylpropion) • Mephedrone • Methedrone
Entactogens: Ethylone • MethylonePhenylisobutylamines Phenylalkylpyrrolidines Stimulants: α-PBP • α-PPP • α-PVP • MDPBP • MDPPP • MDPV • MOPPP • MPBP • MPHP • MPPP • Naphyrone • PEP • Prolintane • PyrovaleroneCatecholamines
(and relatives..)6-FNE • 6-OHDA • α-Me-DA • α-Me-TRA • Adrenochrome • Ciladopa • D-DOPA (Dextrodopa) • Dopamine • Epinephrine (Adrenaline) • Epinine • Fenclonine • Ibopamine • L-DOPA (Levodopa) • L-DOPS (Droxidopa) • L-Phenylalanine • L-Tyrosine • meta-Octopamine • meta-Tyramine • Metanephrine • Metirosine • Methyldopa • Nordefrin (Levonordefrin) • Norepinephrine (Noradrenaline) • Normetanephrine • para-Octopamine • para-TyramineMiscellaneous Amidephrine • Arbutamine • Cafedrine • Denopamine • Dobutamine • Dopexamine • Etafedrine • Ethylnorepinephrine • Etilefrine • Famprofazone • Gepefrine • Isoprenaline (Isoproterenol) • Isoetarine • Metaraminol • Metaterol • Methoxamine • Norfenefrine • Orciprenaline • Phenylephrine (Neosynephrine) • Phenoxybenzamine • Prenalterol • Pronethalol • Propranolol • Salbutamol (Albuterol; Levosalbutamol) • Synephrine (Oxedrine) • Theodrenaline • XamoterolCategories:- Alkaloids
- Cathinones
- Euphoriants
Wikimedia Foundation. 2010.
