- CCL2
-
For the ICAO airport code see Candle Lake Airpark, for the diradical compound see Dichlorocarbene.
Chemokine (C-C motif) ligand 2 (CCL2) also known as monocyte chemotactic protein-1 (MCP-1) or small inducible cytokine A2 is a protein that in humans is encoded by the CCL2 gene. CCL2 is a small cytokine belonging to the CC chemokine family. CCL2 recruits monocytes, memory T cells, and dendritic cells to sites of tissue injury, infection, and inflammation.[1][2]
Contents
Genomics
As with many other CC chemokines, CCL2 is located on chromosome 17 (17q11.2-q21.1) in humans.[3]The gene spans 1,927 bases and lies on the Watson (plus) strand. The gene has three exons and two introns. It is produced as a protein precursor containing signal peptide of 23 amino acids and a mature peptide of 76 amino acids.[4][5] The predicted weight is 11.025 kiloDaltons (kDa).
In the mouse the homolog is Sig-je.
Population genetics
The levels of this protein vary considerably between normal people. Multivariable-adjusted heritability of CCL2 concentrations in whites of European descent has been reported to be 0.37 in plasma and 0.44 in serum[6][7]
Molecular biology
It is a monomeric polypeptide, with a molecular weight of approximately 13kDa. It is tethered on endothelial cells by glycosaminoglycan side chains of proteoglycans. It is primarily secreted by monocytes, macrophages and dendritic cells. It is a platelet derived growth factor inducible gene. It is cleaved by the metalloproteinase MMP-12.
The cell surface receptors that bind CCL2 are CCR2 and CCR4.[8]
This cytokine displays chemotactic activity for monocytes and basophils but not for neutrophils or eosinophils. Deletion of the N-terminal residue converts it from an activator of basophils to an eosinophil chemoattractant. CCL2 causes the degranulation of basophils and mast cells, an effect potentiated by pre-treatment with IL-3 and other cytokines.[9][10] It augments monocyte anti-tumor activity and is essential for granuloma formation
It is found at the site of tooth eruption and bone degradation. In the bone, CCL2 is expressed by mature osteoclasts and osteoblasts and is under the control of nuclear factor κB (NFκB). In human osteoclasts, it has been shown that CCL2 and RANTES (regulated on activation normal T cell expressed and secreted) are unregulated by RANKL (receptor activator of NFκB ligand).[citation needed] Both MCP-1 and RANTES were also shown to induce the formation of TRAP-positive, multinuclear cells from M-CSF-treated monocytes in the absence of RANKL, but produced osteoclasts that lacked cathepsin K expression and resorptive capacity. It is proposed that CCL2 and RANTES act as autocrine loop in human osteoclast differentiation.[11]
The CCL2 chemokine is also expressed by neurons, astrocytes and microglia in nervous tissue. Neuronal expression of CCL2 is mainly found in the cerebral cortex, globus pallidus, hippocampus, paraventricular and supraoptic hypothalamic nuclei, lateral hypothalamus, substantia nigra, facial nuclei, motor and spinal trigeminal nuclei, gigantocellular reticular nucleus and in Purkinje cells in the cerebellum.[12]
Clinical importance
CCL2 has been implicated in the pathogenesis of diseases characterized by monocytic infiltrates, like psoriasis, rheumatoid arthritis and atherosclerosis.[13]
Administration of anti-CCL2 antibodies in a model of glomerulonephritis have shown reduced macrophage and T cell infiltration, as well as reduced crescent formation, scarring and renal impairment.[14]
CCL2 is involved in the neuroinflammatory processes that takes place in various central nervous system (CNS) diseases characterized by neuronal degeneration.[15] Its expression by glial cells is increased in epilepsy,[16][17] brain ischemia[11] Alzheimer’s disease[18] experimental autoimmune encephalomyelitis (EAE),[19] and traumatic brain injury.[20]
Hypomethylation of CpG sites in the CCL2 promoter region may be affected by blood glucose and TG, which then increase the serum CCL2 level and may play a role in the vascular complications of type 2 diabetes[21]
CCL2 induces amylin expression through ERK1/ERK2/JNK-AP1 and NF-κB related signaling pathways independent of CCR2. Amylin upregulation by CCL2 may contribute to elevation of plasma amylin in obesity and insulin resistance.[22]
Adipocytes secrete various adipokines that may be involved in the negative cross-talk between adipose tissue and skeletal muscle. CCL2 impairs insulin signaling in skeletal muscle cells via ERK1/2 activation at doses similar to its physiological plasma concentrations (200 pg/mL), but does not involve activation of the NF-κB pathway. CCL2 significantly reduced insulin-stimulated glucose uptake in myocytes. CCL2 may represent a molecular link in the negative cross-talk between adipose tissue and skeletal muscle assigning a completely novel important role to CCL2 besides inflammation.[23]
Incubation of HL-1 cardiomyocytes and human myocytes with oxidized-LDL induced the expression of BNP and CCL2 genes, while native LDL (N-LDL) had no effect.[24]
Treatment with melatonin in old mice with age related liver inflammation decreased the mRNA expression of TNF-α, IL-1β, HO (HO-1 and HO-2), iNOS, CCL2, NF-κB1, NF-κB2 and NKAP in old male mice. The protein expression of TNF-α, IL-1β was also decreased and IL-10 increased with melatonin treatment. Exogenous administration of melatonin was able to reduce inflammation.[25]
References
- ^ Carr, M. W.; Roth, S. J.; Luther, E.; Rose, S. S.; Springer, T. A. (1994). "Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant". Proceedings of the National Academy of Sciences of the United States of America 91 (9): 3652–3656. PMC 43639. PMID 8170963. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=43639.
- ^ Xu, L. L.; Warren, M. K.; Rose, W. L.; Gong, W.; Wang, J. M. (1996). "Human recombinant monocyte chemotactic protein and other C-C chemokines bind and induce directional migration of dendritic cells in vitro". Journal of leukocyte biology 60 (3): 365–371. PMID 8830793.
- ^ Mehrabian, M.; Sparkes, R. S.; Mohandas, T.; Fogelman, A. M.; Lusis, A. J. (1991). "Localization of monocyte chemotactic protein-1 gene (SCYA2) to human chromosome 17q11.2-q21.1". Genomics 9 (1): 200–203. PMID 2004761.
- ^ Yoshimura, T.; Yuhki, N.; Moore, S. K.; Appella, E.; Lerman, M. I.; Leonard, E. J. (1989). "Human monocyte chemoattractant protein-1 (MCP-1). Full-length cDNA cloning, expression in mitogen-stimulated blood mononuclear leukocytes, and sequence similarity to mouse competence gene JE". FEBS letters 244 (2): 487–493. PMID 2465924.
- ^ Furutani, Y.; Nomura, H.; Notake, M.; Oyamada, Y.; Fukui, T.; Yamada, M.; Larsen, C. G.; Oppenheim, J. J. et al. (1989). "Cloning and sequencing of the cDNA for human monocyte chemotactic and activating factor (MCAF)". Biochemical and biophysical research communications 159 (1): 249–255. PMID 2923622.
- ^ McDermott, D. H.; Yang, Q.; Kathiresan, S.; Cupples, L. A.; Massaro, J. M.; Keaney Jr, J. F.; Larson, M. G.; Vasan, R. S. et al. (2005). "CCL2 Polymorphisms Are Associated with Serum Monocyte Chemoattractant Protein-1 Levels and Myocardial Infarction in the Framingham Heart Study". Circulation 112 (8): 1113–1120. doi:10.1161/CIRCULATIONAHA.105.543579. PMID 16116069.
- ^ Bielinski, S. J.; Pankow, J. S.; Miller, M. B.; Hopkins, P. N.; Eckfeldt, J. H.; Hixson, J.; Liu, Y.; Register, T. et al. (2007). "Circulating MCP-1 levels shows linkage to chemokine receptor gene cluster on chromosome 3: The NHLBI Family Heart Study follow-up examination". Genes and Immunity 8 (8): 684–690. doi:10.1038/sj.gene.6364434. PMID 17917677.
- ^ Craig, M. J.; Loberg, R. D. (2006). "CCL2 (Monocyte Chemoattractant Protein-1) in cancer bone metastases". Cancer and Metastasis Reviews 25 (4): 611–619. doi:10.1007/s10555-006-9027-x. PMID 17160712.
- ^ Conti, P.; Boucher, W.; Letourneau, R.; Feliciani, C.; Reale, M.; Barbacane, R. C.; Vlagopoulos, P.; Bruneau, G. et al. (1995). "Monocyte chemotactic protein-1 provokes mast cell aggregation and 3H5HT release". Immunology 86 (3): 434–440. PMC 1383948. PMID 8550082. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1383948.
- ^ Bischoff, S. C.; Krieger, M.; Brunner, T.; Dahinden, C. A. (1992). "Monocyte chemotactic protein 1 is a potent activator of human basophils". The Journal of experimental medicine 175 (5): 1271–1275. PMC 2119199. PMID 1569397. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2119199.
- ^ a b Kim, M. S.; Day, C. J.; Morrison, N. A. (2005). "MCP-1 is Induced by Receptor Activator of Nuclear Factor- B Ligand, Promotes Human Osteoclast Fusion, and Rescues Granulocyte Macrophage Colony-stimulating Factor Suppression of Osteoclast Formation". Journal of Biological Chemistry 280 (16): 16163–16169. doi:10.1074/jbc.M412713200. PMID 15722361.
- ^ Banisadr, G.; Gosselin, R. D.; Mechighel, P.; Kitabgi, P.; Rostène, W.; Parsadaniantz, S. P. M. L. (2005). "Highly regionalized neuronal expression of monocyte chemoattractant protein-1 (MCP-1/CCL2) in rat brain: Evidence for its colocalization with neurotransmitters and neuropeptides". The Journal of Comparative Neurology 489 (3): 275–292. doi:10.1002/cne.20598. PMID 16025454.
- ^ Xia, M.; Sui, Z. (2009). "Recent developments in CCR2 antagonists". Expert Opinion on Therapeutic Patents 19 (3): 295–303. doi:10.1517/13543770902755129. PMID 19441905.
- ^ Lloyd, C. M.; Minto, A. W.; Dorf, M. E.; Proudfoot, A.; Wells, T. N.; Salant, D. J.; Gutierrez-Ramos, J. C. (1997). "RANTES and Monocyte Chemoattractant Protein–1 (MCP-1) Play an Important Role in the Inflammatory Phase of Crescentic Nephritis, but Only MCP-1 is Involved in Crescent Formation and Interstitial Fibrosis". The Journal of experimental medicine 185 (7): 1371–1380. PMC 2196251. PMID 9104823. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2196251.
- ^ Gerard, C.; Rollins, B. J. (2001). "Chemokines and disease". Nature Immunology 2 (2): 108–115. doi:10.1038/84209. PMID 11175802.
- ^ Foresti, M. L.; Arisi, G. M.; Katki, K.; Montañez, A.; Sanchez, R. M.; Shapiro, L. A. (2009). "Chemokine CCL2 and its receptor CCR2 are increased in the hippocampus following pilocarpine-induced status epilepticus". Journal of Neuroinflammation 6: 40. doi:10.1186/1742-2094-6-40. PMC 2804573. PMID 20034406. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2804573.
- ^ Fabene, P. F.; Bramanti, P.; Constantin, G. (2010). "The emerging role for chemokines in epilepsy". Journal of Neuroimmunology 224 (1–2): 22–27. doi:10.1016/j.jneuroim.2010.05.016. PMID 20542576.
- ^ Hickman, S. E.; El Khoury, J. (2010). "Mechanisms of mononuclear phagocyte recruitment in Alzheimer's disease". CNS & neurological disorders drug targets 9 (2): 168–173. PMID 20205643.
- ^ Ransohoff, R. M.; Hamilton, T. A.; Tani, M.; Stoler, M. H.; Shick, H. E.; Major, J. A.; Estes, M. L.; Thomas, D. M. et al. (1993). "Astrocyte expression of mRNA encoding cytokines IP-10 and JE/MCP-1 in experimental autoimmune encephalomyelitis". The FASEB journal : official publication of the Federation of American Societies for Experimental Biology 7 (6): 592–600. PMID 8472896.
- ^ Semple, B. D.; Bye, N.; Rancan, M.; Ziebell, J. M.; Morganti-Kossmann, M. C. (2009). "Role of CCL2 (MCP-1) in traumatic brain injury (TBI): Evidence from severe TBI patients and CCL2−/− mice". Journal of Cerebral Blood Flow & Metabolism 30 (4): 769–782. doi:10.1038/jcbfm.2009.262. PMC 2949175. PMID 20029451. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2949175.
- ^ Liu, Z. H.; Chen, L. L.; Deng, X. L.; Song, H. J.; Liao, Y. F.; Zeng, T. S.; Zheng, J.; Li, H. Q. (2011). "Methylation Status of CpG Sites in the MCP-1 Promoter is Correlated to Serum MCP-1 in type 2 Diabetes". Journal of endocrinological investigation. doi:10.3275/7981. PMID 21975431.
- ^ Cai, K.; Qi, D.; Hou, X.; Wang, O.; Chen, J.; Deng, B.; Qian, L.; Liu, X. et al. (2011). Fadini, Gian Paolo. ed. "MCP-1 Upregulates Amylin Expression in Murine Pancreatic β Cells through ERK/JNK-AP1 and NF-κB Related Signaling Pathways Independent of CCR2". PLoS ONE 6 (5): e19559. doi:10.1371/journal.pone.0019559. PMC 3092759. PMID 21589925. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3092759.
- ^ Sell, H.; Dietze-Schroeder, D.; Kaiser, U.; Eckel, J. (2006). "Monocyte Chemotactic Protein-1 is a Potential Player in the Negative Cross-Talk between Adipose Tissue and Skeletal Muscle". Endocrinology 147 (5): 2458–2467. doi:10.1210/en.2005-0969. PMID 16439461.
- ^ Chandrakala, A. N.; Sukul, D.; Selvarajan, K.; Sai-Sudhakar, C.; Sun, B.; Parthasarathy, S. (2011). "Induction of Brain Natriuretic Peptide and Monocyte Chemotactic Protein1 Gene Expressions by Oxidized Lowdensity Lipoprotein-Relevance to Ischemic Heart Failure". AJP: Cell Physiology. doi:10.1152/ajpcell.00116.2011. PMID 21900689.
- ^ Cuesta, S.; Kireev, R.; Forman, K.; García, C.; Escames, G.; Ariznavarreta, C.; Vara, E.; Tresguerres, J. S. A. F. (2010). "Melatonin improves inflammation processes in liver of senescence-accelerated prone male mice (SAMP8)". Experimental Gerontology 45 (12): 950–956. doi:10.1016/j.exger.2010.08.016. PMID 20817086.
Further reading
- Yoshimura, T.; Leonard, E. J. (1991). "Human monocyte chemoattractant protein-1 (MCP-1)". Advances in experimental medicine and biology 305: 47–56. PMID 1661560.
- Wahl, S. M.; Greenwell-Wild, T.; Hale-Donze, H.; Moutsopoulos, N.; Orenstein, J. M. (2000). "Permissive factors for HIV-1 infection of macrophages". Journal of leukocyte biology 68 (3): 303–310. PMID 10985244.
- Sell, H.; Eckel, J. R. (2007). "Monocyte chemotactic protein-1 and its role in insulin resistance". Current Opinion in Lipidology 18 (3): 258–262. doi:10.1097/MOL.0b013e3281338546. PMID 17495598.
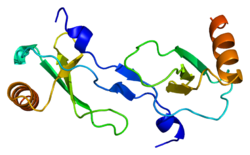
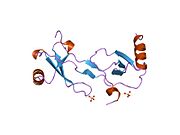
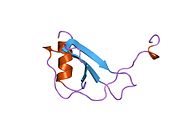
PDB gallery 1dok: MONOCYTE CHEMOATTRACTANT PROTEIN 1, P-FORM1dol: MONOCYTE CHEMOATTRACTANT PROTEIN 1, I-FORM1dom: SOLUTION STRUCTURE OF THE MONOCYTE CHEMOATTRACTANT PROTEIN-1 DIMER USING HETERONUCLEAR, NMR, MINIMIZED AVERAGE STRUCTURE1don: SOLUTION STRUCTURE OF THE MONOCYTE CHEMOATTRACTANT PROTEIN-1 DIMER USING HETERONUCLEAR, NMR, 20 STRUCTURES1ml0: VIRAL CHEMOKINE BINDING PROTEIN M3 FROM MURINE GAMMAHERPESVIRUS68 IN COMPLEX WITH THE P8A VARIANT OF CC-CHEMOKINE MCP-12bdn: Crystal structure of human MCP-1 bound to a blocking antibody, 11K2Cell signaling: cytokines By family CCLCXCLCX3CLXCLIL6 like/gp130IL-12 family/IL12RB1OtherIL-10 familyIL-17 familyOtherBy function/
cellB trdu: iter (nrpl/grfl/cytl/horl), csrc (lgic, enzr, gprc, igsr, intg, nrpr/grfr/cytr), itra (adap, gbpr, mapk), calc, lipd; path (hedp, wntp, tgfp+mapp, notp, jakp, fsap, hipp, tlrp) Categories:- Human proteins
- Chromosome 17 gene stubs
- Cytokines
Wikimedia Foundation. 2010.