- Alizarin
-
"Madder lake" redirects here. For the Australian band, see Madder Lake (band).
Alizarin 
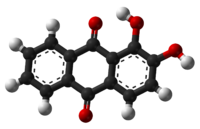
 1,2-dihydroxy-9,10-anthracenedioneOther names1,2-Dihydroxyanthraquinone, Turkey red, Mordant red 11, Alizarin B, Alizarin red
1,2-dihydroxy-9,10-anthracenedioneOther names1,2-Dihydroxyanthraquinone, Turkey red, Mordant red 11, Alizarin B, Alizarin redIdentifiers CAS number 72-48-0 
PubChem 6293 ChemSpider 6056 
UNII 60MEW57T9G 
KEGG C01474 
ChEBI CHEBI:16866 
ChEMBL CHEMBL55814 
Jmol-3D images Image 1 - O=C2c1ccccc1C(=O)c3c2ccc(O)c3O
Properties Molecular formula C14H8O4 Molar mass 240.21 g mol−1 Appearance orange-red crystals or powder Density 1.540 g/cm3 Melting point 279–83 °C
Boiling point 430 °C
Solubility in water slightly to sparingly soluble Acidity (pKa) 6.94 Hazards MSDS External MSDS R-phrases R36 R37 R38 S-phrases S26 S36 Related compounds Related compounds anthraquinone, anthracene  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Alizarin or 1,2-dihydroxyanthraquinone (also known as Mordant Red 11 and Turkey Red[1]) is an organic compound with formula C14H8O4 that has been used throughout history as a prominent dye, originally derived from the roots of plants of the madder genus.
Alizarin was used as a red dye for the English parliamentary "new model" army. The distinctive red color would continue to be worn for centuries, giving English and later British soldiers the nickname of "redcoat". In 1869, it became the first natural pigment to be duplicated synthetically.[2]
Different dyes were used by the British, at different times, as one dye name that is commonly cited is "cochineal". Cochineal is derived from several related species of scale insect, endemic to South America.
Alizarin is the main ingredient for the manufacture of the madder lake pigments known to painters as Rose madder and Alizarin crimson. The term is also part of the name for a variety of related dyes, such as Alizarine Cyanine Green G and Alizarine Brilliant Blue R, and gave its name to alizarin crimson, a particular shade of red. The word derives from the Arabic al-usara "juice".[3]
Contents
Occurrence
Alizarin occurs in the root of the common madder (Rubia tinctorum) and in various parts of Indian madder (Rubia cordifolia).[4]
History
Madder has been cultivated as a dyestuff since antiquity in central Asia and Egypt, where it was grown as early as 1500 BC. Cloth dyed with madder root pigment was found in the tomb of the Pharaoh Tutankhamun and in the ruins of Pompeii and ancient Corinth. In the Middle Ages, Charlemagne encouraged madder cultivation. It grew well in the sandy soils of the Netherlands and became an important part of the local economy.
 alizarin color
alizarin colorBy 1804, the English dye maker George Field[5] had refined the technique to lake madder by treating it with alum, and an alkali,[6] that converts the water-soluble madder extract into a solid, insoluble pigment. This resulting madder lake has a longer-lasting color, and can be used more efficaciously, for example by blending it into a paint. Over the following years, it was found that other metal salts, including those containing iron, tin, and chromium, could be used in place of alum to give madder-based pigments of various other colors. This general method of preparing lakes has been known for centuries.[7]
In 1826, the French chemist Pierre-Jean Robiquet found that madder root contained two colorants, the red alizarin and the more rapidly fading purpurin. The alizarin component became the first natural dye to be synthetically duplicated in 1868 when the German chemists Carl Graebe and Carl Liebermann, working for BASF, found a way to produce it from anthracene. About the same time, the English dye chemist William Henry Perkin independently discovered the same synthesis, although the BASF group filed their patent before Perkin by only one day. The subsequent discovery (made by Broenner and Gutzhow in 1871) that anthracene could be abstracted from coal tar further advanced the importance and affordability of alizarin's artificial synthesis.[8]
The synthetic alizarin could be produced for a fraction of the cost of the natural product, and the market for madder collapsed virtually overnight. The principal synthesis entailed oxidation of anthraquinone-2-sulfonic acid with sodium nitrate in concentrated sodium hydroxide. Alizarin itself has been in turn largely replaced today by the more light-resistant quinacridone pigments developed at DuPont in 1958.
Structure and properties
Alizarin is one of ten dihydroxyanthraquinone isomers. Its molecular structure can be viewed as being derived from anthraquinone by replacement of two neighboring hydrogen atoms (H) by hydroxyl groups (-OH).
It is soluble in hexane and chloroform, and can be obtained from the latter as red-purple crystals, m.p. 277–278 °C. [4]
Alizarin changes color depending on the pH of the solution it is in, thereby making it a pH indicator.[9]
Applications
Alizarin Red is used in a biochemical assay to determine, quantitatively by colorimetry, the presence of calcific deposition by cells of an osteogenic lineage. As such it is an early stage marker (days 10–16 of in vitro culture) of matrix mineralization, a crucial step towards the formation of calcified extracellular matrix associated with true bone.[citation needed]
Alizarin's abilities as a biological stain were first noted in 1567, when it was observed that when fed to animals, it stained their teeth and bones red. The chemical is now commonly used in medical studies involving calcium. Free (ionic) calcium forms precipitates with alizarin, and tissue block containing calcium stain red immediately when immersed in alizarin. Thus, both pure calcium and calcium in bones and other tissues can be stained. The process of staining calcium with alizarin works best when conducted in basic solution. [10]
In clinical practice, it is used to stain synovial fluid to assess for basic calcium phosphate crystals.[11] Alizarin has also been used in studies involving bone growth, osteoporosis, bone marrow, calcium deposits in the vascular system, cellular signaling, gene expression, tissue engineering, and mesenchymal stem cells.[12]
In geology, it is used as a stain to indicate the calcium carbonate minerals, calcite and aragonite.[13]
See also
- Alizarine ink
- Aniline
- 1,2,4-Trihydroxyanthraquinone or purpurin, another red dye that occurs in madder root.
- Hydroxyanthraquinone
- Dihydroxyanthraquinone
- List of dyes
- List of colors (compact)
References
- ^ SigmaAldrich Catalog: Alizarin
- ^ Hans-Samuel Bien, Josef Stawitz, Klaus Wunderlich “Anthraquinone Dyes and Intermediates” in Ullmann’s Encyclopedia of Industrial Chemistry 2005 Wiley-VCH, Weinheim: 2005. doi:10.1002/14356007.a02 355.
- ^ alizarin. Dictionary.com. Dictionary.com Unabridged (v 1.1). Random House, Inc. http://dictionary.reference.com/browse/alizarin (accessed: January 02, 2007).
- ^ a b Padma S. Vankar, Rakhi Shanker, Debajit Mahanta and S.C. Tiwari (2008), Ecofriendly sonicator dyeing of cotton with Rubia cordifolia Linn. using biomordant. Dyes and Pigments, Volume 76, Issue 1, , Pages 207-212. doi:10.1016/j.dyepig.2006.08.023
- ^ Field's notes are held at the Courtauld Institute of Art. See: http://www.aim25.ac.uk/cgi-bin/search2?coll_id=4107&inst_id=2 (accessed: 2007/09/05)
- ^ Winsor Newton's madder pigment is made according to his process. See http://www.winsornewton.com/artnews/EN/artnewsletterA4_english03_2002.pdf page 6. (accessed: 2007/09/03). Note that Henry Charles Newton, founder of Winsor Newton, was his assistant and friend.
- ^ Daniel V. Thompson – The Materials and Techniques of Medieval Painting – Dover – pp115–124. ISBN 0-486-20327-1
- ^ Broenner, J; Gutzhow, H (1871). "Process for preparing anthracene from coal-tar pitch, and preparation of dye-stuffs from anthracene". Dinglers Polytechnisches Journal: 545.
- ^ Meloan, SN; Puchtler, H; Valentine, LS (1972). "Alkaline and acid alizarin red S stains for alkali-soluble and alkali-insoluble calcium deposits". Journal of Histochemistry and Cytochemistry 93: 190–197.
- ^ Puchtler, H; Meloan, SN; Terry, MS (1969). "On the history and mechanism of alizarin red s stains for calcium". The Journal of Histochemistry and Cytochemistry 17: 110–124.
- ^ Paul H; Reginato AJ, Schumacher HR. "Alizarin red S staining as a screening test to detect calcium compounds in synovial fluid". Arthritis and rheumatism 26 (2): 191–200. PMID 6186260.
- ^ Puchtler, H; Meloan, SN; Terry, MS (1969). "On the history and mechanism of alizarin red s stains for calcium". The Journal of Histochemistry and Cytochemistry 17: 110–124.
- ^ Green, Owen R. (2001). A manual of practical laboratory and field techniques in palaeobiology. Springer. p. 56. ISBN 978-0-412-58980-5. http://books.google.com/books?id=4lJr5LwvnX8C.
External links
Hydroxyanthraquinones |Dihydroxyanthraquinones Alizarin | Aloe emodin | Damnacanthal | 1,3-Dihydroxyanthraquinone | 1,4-Dihydroxyanthraquinone | 1,8-Dihydroxyanthraquinone | RheinTrihydroxyanthraquinones Tetrahydroxyanthraquinones 1,2,4-Trihydroxyanthraquinone | 1,3,8-TrihydroxyanthraquinonePentahydroxyanthraquinones |Hexahydroxyanthraquinones |Heptahydroxyanthraquinones |Misc: Anthraquinone drugs | Anthraquinone dyes Carminic acid | 2-Ethylanthraquinone | Quinalizarin | Rufigallol | Senna glycosides | Sodium 2-anthraquinonesulfonateCategories:- Anthraquinone dyes
- Dihydroxyanthraquinones
- Catechols
- Organic pigments
Wikimedia Foundation. 2010.
