- Calcium oxalate
-
Calcium oxalate 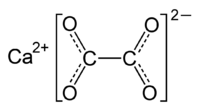 calcium ethanedioate
calcium ethanedioateIdentifiers CAS number 25454-23-3  , (anhydrous)
, (anhydrous)
5794-28-5 (monohydrate)PubChem 16212978 ChemSpider 30549 
ChEBI CHEBI:60579 
Jmol-3D images Image 1 - C(=O)(C(=O)[O-])[O-].[Ca+2]
Properties Molecular formula CaC2O4 Molar mass 128.097 g/mol, anhydrous
146.112 g/mol, monohydrateAppearance white solid Density 2.12 g/cm³, anhydrous
2.12 g/cm³, monohydrateMelting point 200°C, decomposes (monohydrate)
Solubility in water 0.00067 g/100 ml (20 °C)  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Calcium oxalate (in archaic terminology, oxalate of lime) is a chemical compound that forms needle-shaped crystals, known in plants as raphides. A major constituent of human kidney stones, the chemical is also found in beerstone, a scale that forms on containers used in breweries. Its chemical formula is CaC2O4 or Ca (COO)2.
Contents
Occurrence
Quantities of calcium oxalate are found in many tropical house plants. Calcium oxalate is a poisonous substance that can produce sores and numbing on ingestion and could even be fatal. The poisonous plant dumb cane (Dieffenbachia) contains the substance and on ingestion can prevent speech and be suffocating. It is also found in rhubarb (in large quantities in the leaves) and in species of Oxalis, Araceae, taro, kiwifruit, tea leaves, agaves, and Alocasia and in spinach in varying amounts. Insoluble calcium oxalate crystals are found in plant stems, roots, and leaves and produced in idioblasts. Kidney stone sufferers should not eat plants high in oxalates.
Calcium oxalate, as 'beerstone', is a brownish precipitate that tends to accumulate within vats, barrels and other containers used in the brewing of beer. If not completely removed in a cleaning process, beerstone will leave an unsanitary surface that can harbour microorganisms.[1] Beerstone is composed of calcium and magnesium salts and various organic compounds left over from the brewing process; it promotes the growth of unwanted microorganisms that can adversely affect or even ruin the flavor of a batch of beer.
Calcium oxalate crystals in the urine are the most common constituent of human kidney stones, and calcium oxalate crystal formation is also one of the toxic effects of ethylene glycol poisoning.
Hydrated forms of the compound occur naturally as three mineral species: whewellite (monohydrate, known from some coal beds), weddellite (dihydrate) and a very rare trihydrate called caoxite.
Effects of Ingestion
Even a small dose of calcium oxalate is enough to cause intense sensations of burning in the mouth and throat, swelling, and choking that could last for up to two weeks.[2] In greater doses it can cause severe digestive upset, breathing difficulties, coma or even death. Recovery from severe oxalate poisoning is possible, but permanent liver and kidney damage may have occurred.
The stalks of plants in the Dieffenbachia genus produce the most severe oxalate reactions. The needle-like oxalate crystals produce pain and swelling when they contact lips, tongue, oral mucosa, conjunctiva, or skin. Edema primarily is due to direct trauma from the needle-like crystals and, to a lesser extent, by other plant toxins (e.g., bradykinins, enzymes).
Depending on the plant ingested, mild (Elephant Ear Colocasia esculenta) to more severe (Jack in the Pulpit, Arisaema) can cause compromised airways. One bite on the Arisaema seed pod will result in immediate swelling and burning. It will take over 12 hours for the swelling to subside.[citation needed]
Treatment
Medication administered at the ER may include diphenhydramine, epinephrine, or famotidine, all intravenously. Although this most likely will be a localized reaction, it will be treated by the ER as an anaphylactic reaction.[citation needed]
References
- ^ Johnson, Dana (23 March 1998). "Removing Beerstone". Modern Brewery Age. Birko Corporation R&D. http://www.birkocorp.com/Brewing/beerstone.html. Retrieved 2007-08-06.
- ^ Outbreak of Food-borne Illness Associated with Plant Material Containing Raphides. Informa Healthcare.
See also
Calcium compounds CaB6 · CaBr2 · CaC2 · CaCN2 · CaCO3 · CaC2O4 · CaCl · CaCl2 · Ca(ClO)2 · Ca(ClO3)2 · CaCrO4 · CaF2 · CaH2 · Ca(HCO3)2 · CaH2S2O6 · CaI2 · Ca(IO3)2 · Ca(MnO4)2 · Ca(NO3)2 · CaO · CaO2 · Ca(OH)2 · CaS · CaSO3 · CaSO4 · CaSi2 · CaTiO3 · Ca2P2O7 · Ca2SiO4 · Ca3(AsO4)2 · Ca3(BO3)2 · Ca3(C6H5O7)2 · Ca3N2 · Ca3P2 · Ca3(PO4)2 · Ca(H2PO4)2 · CaHPO4 · C36H70CaO4
Categories:- Oxalates
- Calcium compounds
- Kidney
Wikimedia Foundation. 2010.